Study on gas phase reaction mechanism of HCN and H2/H2O based on density functional theory
-
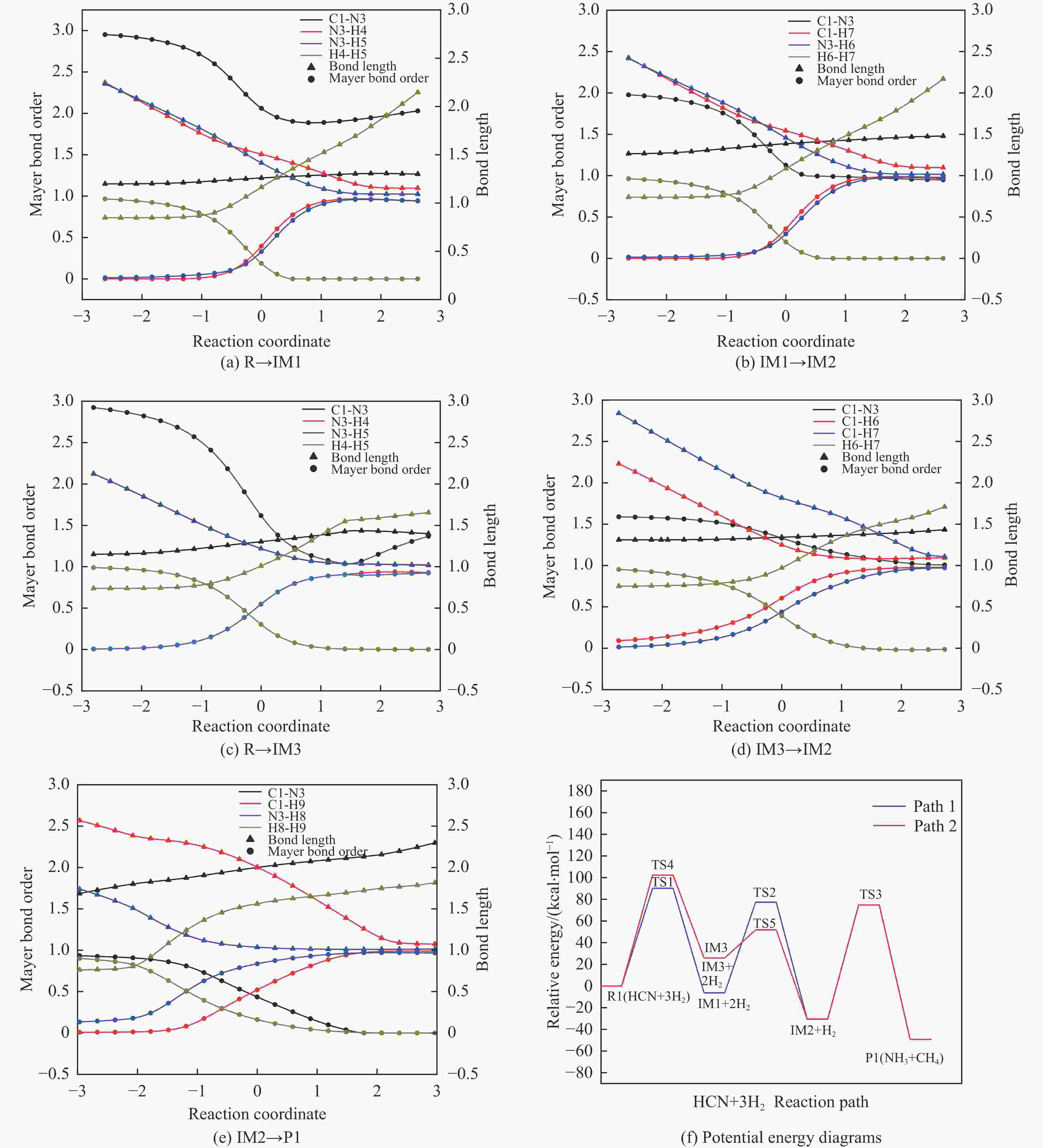
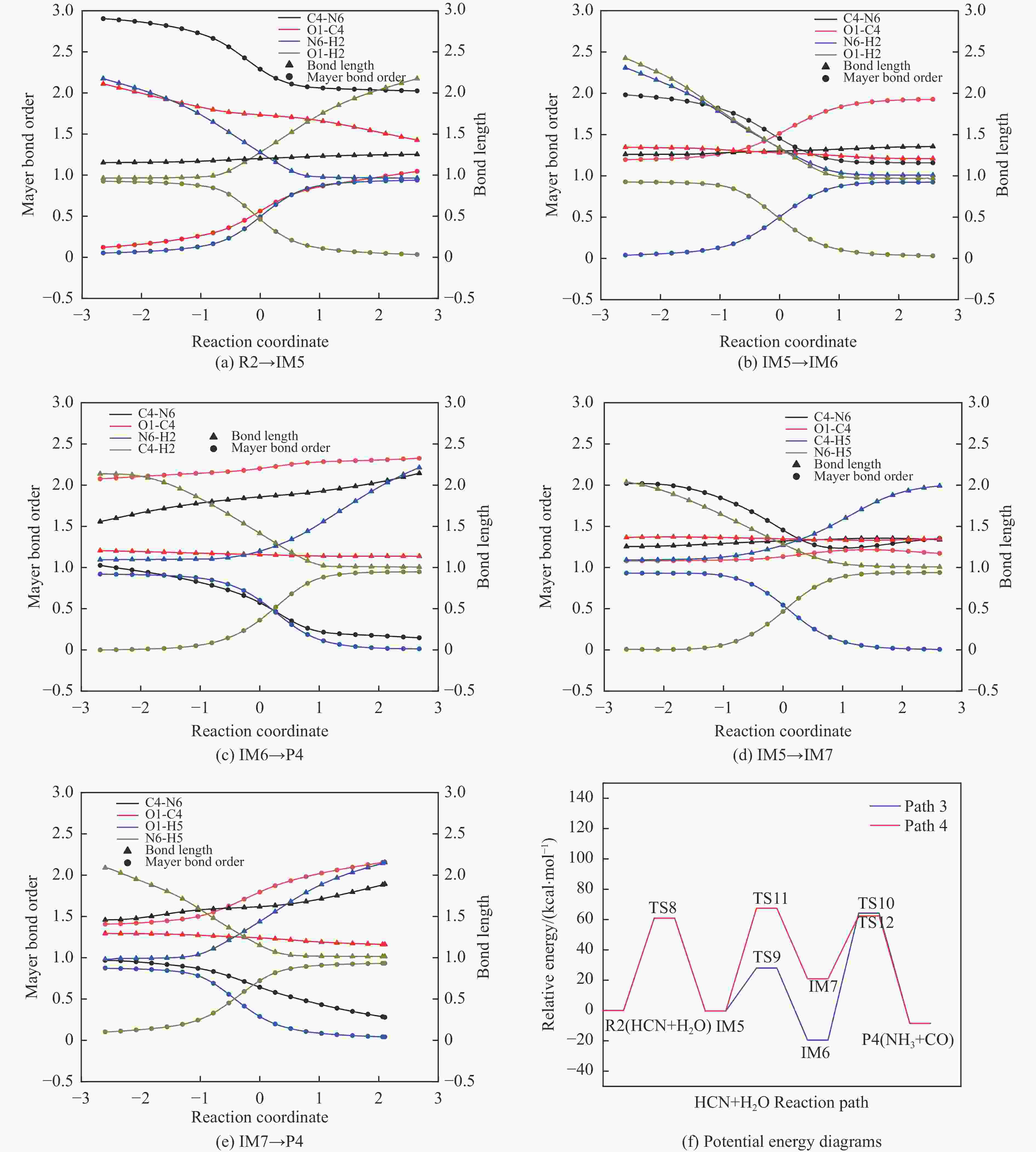
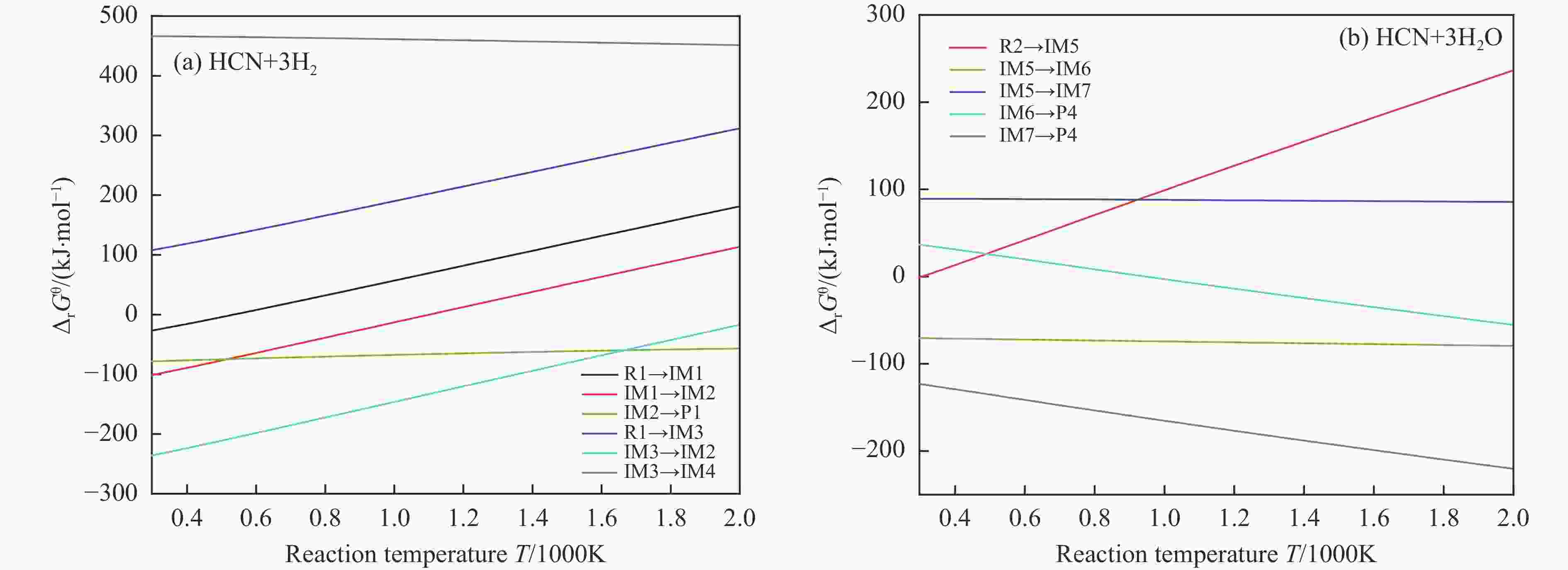
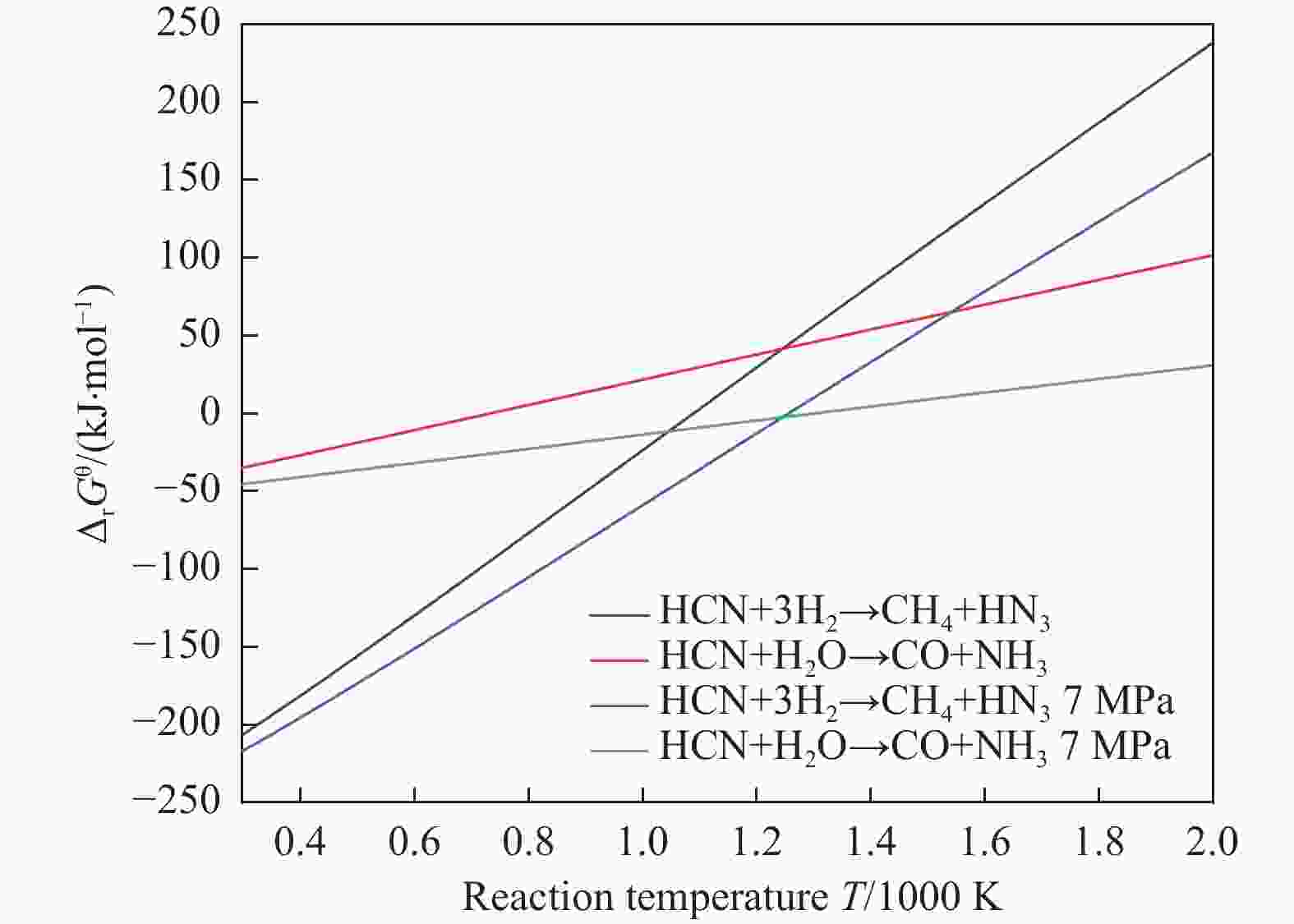
摘要: 含HCN的废弃物在气化炉内的高温转化是其绿色处理的方法之一,其中,HCN与H2/H2O的反应是其在气化炉内的主要转化过程。本工作基于密度泛函理论,采用Gaussian及其配套软件对HCN与H2/H2O的反应机理进行了研究。通过分子成键、断键角度提出HCN与H2/H2O的各两种反应路径,结合能垒和热力学分析确定了相对最优路径,并计算了相对最优反应路径的速率常数。结果表明,HCN与H2反应相对最优路径为:三个H2分子在C≡N上分三步进行加成得到产物CH4+NH3;HCN与H2O反应相对最优路径为:H2O分子进攻C原子,O原子和C原子的H先后转移至N原子得到产物CO+NH3。两条相对最优路径在1473 K以上有明显反应速率,分别为9.57×10−4和1.71 mol/(L·s)。研究结果为高温下HCN与H2/H2O反应的工艺和设备开发提供了理论数据支撑。Abstract: HCN is a highly toxic substance that can enter the human body through the skin and respiratory system, and in severe cases, cause death. HCN can achieve partial conversion under high-temperature gasification conditions, mainly by reacting with H2 and H2O. In order to further explore the micro reaction mechanism of HCN with H2 and H2O during gasification, and to investigate the effects of temperature and pressure changes on the reaction, this paper uses quantum chemical simulation methods to study the reaction path, reaction thermodynamics, and kinetics of the above reactions, and quantitatively analyzes the changes in thermodynamic parameters and reaction rate constants with temperature, fitting the Arrhenius equation related to the reaction. Calculate the distribution of Fukui functions for various reactants and intermediates in the reaction process of HCN with H2 and H2O using Multiwfn, and speculate on possible reaction pathways. The transition state search and single point energy calculation of the reaction process between HCN and H2 and H2O were carried out using Gaussian & Gaussian View. Similarly, using the wave function program Multiwfn to calculate the Mayer bond order. The analysis of the bond order curve during the reaction process can reveal the changes in the strength of chemical bonds and the situation of bond formation and breaking. Use the Shermo program to calculate the thermodynamic parameters of each stagnation point at different temperatures, including enthalpy, entropy, Gibbs free energy, and partition function. Finally, the KiSThelP program was used to calculate the reaction rate constants for each step of the reaction based on classical transition state theory at different temperatures. The results show that the relatively optimal path for the reaction between HCN and H2 is as follows: three H2 molecules are added in three steps on C≡N to obtain the product CH4+NH3; The relatively optimal path for the reaction between HCN and H2O is as follows: H2O molecules attack C atoms, and the H of O and C atoms are transferred to N atoms to obtain the product CO+NH3. The first step of reaction between HCN and H2 is R1→IM1 which is below 534 K with ΔG<0. After exceeding this temperature, ΔG>0 becomes a reverse spontaneous reaction. It can be considered that an increase in temperature is not conducive to the electrophilic addition reaction of the first H2 on C≡N. The second step is IM1→IM2, with ΔG below 1103 K less than 0 and above greater than 0, indicates that the spontaneity of the second step H2 addition reaction is inhibited as the temperature gradually increases. The third step is IM2→P1. Within the set temperature range, its ΔG is always less than 0, and the reaction can always proceed spontaneously. The ΔG of the first step reaction R2→IM5 in Path 3 is only less than 0 at room temperature, indicating that this step is difficult to occur spontaneously at high temperatures. Path 3 second step reaction IM5→IM6 ΔG is always less than 0 within the set temperature range. The third step of the reaction is IM6→P2, and the temperature of ΔG below 958 K is greater than 0, making it difficult to occur spontaneously. The research results on changes in pressure and free energy show that pressure can increase the upper temperature limit for spontaneous reaction.The reaction rates of HCN with H2 and HCN with H2O are relatively fast at high temperatures. The rate determining steps for Path 1 and Path 3 at high temperatures are R1 → IM1, R2 → IM5, respectively. The rate constants for the two reaction steps above 1473 K are 9.57×10−4 and 1.71 mol/(L·s), respectively. The pre-exponential factors for these two reactions were calculated to be 4.45×109 and 4.68×108 s−1, and the activation energies were 357.62 and 239.30 kJ/mol, respectively.
-
Key words:
- HCN /
- gas phase reaction /
- DFT /
- thermodynamics /
- kinetics
-
表 1 HCN与H2反应过程中优化后分子的Fukui函数等值面及各原子位点亲电反应CFF值
Table 1 Optimized Fukui function isosurfaces of molecules and CFF values of electrophilic reactions at various atomic sites during the HCN and H2 reaction process
Species & Fukui function isosurface Atomic number $ {f}_{A}^{-} $ HCN 
HCN-H
HCN-C
HCN-N0.02536
0.38361
0.59106IM1 
IM1-C1
IM1-H2
IM1-N3
IM1-H4
IM1-H50.15909
0.05071
0.63940
0.07323
0.07745IM2 
IM2-C1
IM2-H2
IM2-N3
IM2-H4
IM2-H5
IM2-H6
IM2-H70.10057
0.01700
0.68701
0.05376
0.01703
0.05376
0.07087IM3 
IM3-C1
IM3-H2
IM3-N3
IM3-H4
IM3-H50.75997
0.08608
0.08908
0.04195
0.02294


表 2 HCN与H2反应路径中的反应物、重要的过渡态、中间体和产物的优化几何结构
Table 2 Optimized geometric structures of reactants, important transition states, intermediates, and products in the reaction pathway between HCN and H2
Species Structure parameter Bond length/Å Bond angle/(°) Dihedral angel/(°) H2 
R(1,2) 0.7442 HCN 
R(2,1) 1.0668
R(1,3) 1.1491A(2,1,3) 180.0000 TS1 
R(1,3) 1.0928
R(1,5) 1.5135
R(3,4) 1.3986
R(5,4) 1.1139
R(1,2) 1.0928A(2,1,3) 144.6852
A(3,1,5) 116.0948
A(4,3,1) 55.5995
A(1,5,4) 53.2891D(1,3,4,5) 0.0005
D(2,1,5,4) 179.9998IM1 
R(1,3) 1.2658
R(1,5) 1.0972
R(3,4) 1.0273
R(1,2) 1.0921A(2,1,5) 115.9175
A(2,1,3) 118.7672
A(4,3,1) 110.5457D(5,1,3,4) 0.0001
D(2,1,3,4) −180.0012TS2 
R(1,7) 1.5447
R(3,6) 1.4571
R(1,3) 1.3869
R(7,6) 1.0871A(5,1,3) 123.5767
A(7,1,3) 110.5757
A(6,3,1) 52.7742
A(7,6,3) 142.4171D(7,1,3,6) −1.4737
D(7,1,3,4) 88.6393
D(2,1,3,4) −168.0049
D(6,3,1,5) −110.7991IM2 
R(1,7) 1.1015
R(3,6) 1.0150
R(1,3) 1.4661
R(3,4) 1.0150A(7,1,3) 115.5229
A(4,3,6) 106.2779
A(5,1,3) 109.2559
A(4,3,1) 110.0534D(7,1,3,6) −58.4100
D(7,1,3,4) 58.3957
D(2,1,3,4) −179.9826
D(6,3,1,5) 179.9200TS3 
R(1,9) 2.0063
R(3,8) 1.0370
R(1,3) 2.0039
R(1,5) 1.0886
R(3,6) 1.0275A(9,1,3) 68.9162
A(8,3,1) 89.2010
A(9,8,3) 120.3781
A(7,1,5) 113.7174
A(8,3,4) 107.7419D(9,1,3,8) 14.9600
D(9,1,3,6) 138.9165
D(8,3,1,7) −53.7533
D(7,1,3,6) 69.3032
D(5,1,3,4) 84.2266TS4 
R(1,2) 1.1021
R(1,3) 1.3035
R(3,4) 1.2204
R(3,5) 1.2196
R(4,5) 1.0095A(2,1,3) 117.8227
A(1,3,4) 109.7895
A(1,3,5) 109.8708
A(4,3,5) 48.8790D(2,1,3,4) −21.0180
D(2,1,3,5) 26.1657IM3 
R(1,2) 1.1101
R(1,3) 1.3109
R(3,4) 1.0224
R(3,5) 1.0137A(2,1,3) 105.8954
A(1.3,4) 126.8288
A(1,3,5) 119.4255
A(4,3,5) 110.3356D(2,1,3,4) −0.0485
D(2,1,3,5) −179.9298TS5 
R(1,6) 1.2521
R(1,7) 1.8189
R(6,7) 0.9709
R(1,3) 1.3417A(6,1,7) 30.2755
A(6,1,3) 120.3818
A(7,1,3) 110.7730
A(5,3,4) 115.1700D(6,1,3,4) −110.0514
D(7,1,3,5) 87.9517
D(2,1,3,5) −176.3768
D(2,1,3,4) 17.5926P1 
R(1,7) 1.0919
R(3,4) 1.0150A(4,3,6) 107.2535
A(2,1,5) 109.0833表 3 HCN与H2O反应过程中优化后分子的Fukui函数等值面及各原子位点亲电反应CFF值
Table 3 Optimized Fukui function isosurfaces of molecules and CFF values of electrophilic reactions at various atomic sites during the HCN and H2O reaction process
Species & Fukui function isosurface Atomic number $ {f}_{A}^{-} $ HCN 
HCN-H
HCN-C
HCN-N0.02536
0.38361
0.59106IM5 
IM5-O1
IM5-H2
IM5-H3
IM5-C4
IM5-H5
IM5-N60.18270
0.05485
0.01658
0.14178
0..02315
0..58080IM6 
IM6-O1
IM6-H2
IM6-H3
IM6-C4
IM6-H5
IM6-N60.57988
0.02032
0.02041
0.10906
0.05605
0.21426IM7 
IM7-O1
IM7-H2
IM7-H3
IM7-C4
IM7-H5
IM7-N60.15798
0.03530
0.02008
0.66425
0.01201
0.11044



表 4 HCN与H2O反应路径中的反应物、重要的过渡态、中间体和产物的优化几何结构
Table 4 Optimized geometric structures of reactants, important transition states, intermediates, and products in the reaction pathway between HCN and H2O
Species Structure parameter Bond length/Å Bond angle/(°) Dihedral angel/(°) >H2O 
R(1,2) 0.9619
R(1,3) 0.9619A(2,1,3) 105.0645 HCN 
R(2,1) 1.0668
R(1,3) 1.1491A(2,1,3) 180.0000 TS8 
R(4,1) 1.7315
R(5,2) 1.3527
R(1,2) 1.2767
R(4,6) 1.2022A(5,4,6) 149.9711
A(3,1,2) 113.1294
A(5,4,1) 108.3661
A(4,6,2) 75.8243D(3,1,4,6) 106.5879
D(5,4,1,2) 178.6648
D(1,4,6,2) −1.4199IM5 
R(3,1) 0.9638
R(1,4) 1.3656
R(4,6) 1.2569
R(6,2) 1.0226
R(5,4) 1.0910A(3,1,4) 110.2408
A(5,4,6) 120.5837
A(4,6,2) 111.2873
A(1,4,6) 124.5519D(3,1,4,6) −179.9530
D(5,4,6,2) 179.9971
D(3,1,4,5) 0.0067
D(1,4,6,2) −0.0454TS9 
R(3,6) 1.3419
R(1,4) 1.2799
R(6,4) 1.3009
R(1,3) 1.3343A(1,4,5) 122.7112
A(1,4,6) 108.9032
A(3,6,2) 161.0386
A(3,6,4) 73.4046
A(2,6,4) 125.5567D(5,4,6,3) −179.9966
D(2,6,4,1) 179.9304
D(3,1,4,6) 0.0146IM6 
R(3,6) 1.0084
R(1,4) 1.2094
R(6,4) 1.3608
R(2,6) 1.0058
R(4,5) 1.1078A(1,4,6) 125.0723
A(5,4,6) 111.9086
A(2,6,3) 119.3349
A(3,6,4) 119.0869D(5,4,6,2) 0.0109
D(1,4,6,3) −0.0085TS10 
R(4,6) 1.8572
R(1,4) 1.1567
R(5,6) 1.4147
R(2,6) 1.0179
R(3,6) 1.0188A(1,4,6) 122.1969
A(4,6,3) 123.6470
A(2,6,3) 111.6923
A(5,6,2) 110.1399
A(5,6,3) 136.9573D(2,6,4,1) −110.6570
D(5,4,6,3) −124.9287
D(1,4,6,3) 31.9173
D(2,6,4,5) 92.4970P4 
R(1,4) 1.1278
R(6,5) 1.0151
R(6,3) 1.0154
R(6,2) 1.0154A(5,6,3) 107.0866
A(5,6,2) 106.8252
A(2,6,3) 107.0106TS11 
R(3,1) 0.9637
R(4,6) 1.3231
R(4,1) 1.3483
R(6,2) 1.0402
R(6,5) 1.2844A(3,1,4) 107.9583
A(1,4,6) 115.9444
A(5,6,2) 131.5169
A(4,6,2) 113.6095
A(5,6,4) 58.3649D(3,1,4,6) 179.9985
D(3,1,4,5) −108.0773
D(2,6,4,1) 5.83156
D(2,6,4,5) −125.4769IM7 
R(5,6) 1.0052
R(2,6) 1.0197
R(6,4) 1.3276
R(4,1) 1.3535
R(1,3) 0.9602A(5,6,2) 118.1916
A(5,6,4) 119.0565
A(2,6,4) 122.7519
A(6,4,1) 107.0787
A(4,1,3) 106.4910D(6,4,1,3) 179.9516
D(5,6,4,1) 179.9516
D(2,6,4,1) −0.0131TS12 
R(1,4) 1.2555
R(4,6) 1.6007
R(6,3) 1.3443
R(6,5) 1.0205
R(6,2) 1.0205A(1,4,6) 94.9820
A(3,6,4) 69.2820
A(4,6,5) 112.7127
A(2,6,5) 108.0600
A(2,6,3) 123.4042D(2,6,3,1) −104.1762
D(5,6,4,1) −118.6872
D(1,3,6,4) 0.0111表 5 HCN与H2/H2O热力学可行反应的焓变△H
Table 5 △H of thermodynamically feasible reactions between HCN and H2/H2O
Reaction △H/(kJ·mol−1) T/K R1→IM1 −57.83→−68.03→−65.18 298→1198→1998 IM1→IM2 −136.31→−141.34→−135.06 298→948→1998 IM2→P1 −84→−72.93 298→1998 HCN+3H2→CH4+NH3 −278.15→−290.05→−273.18 298→948→1998 R2→IM5 −766.76→−769.23→−756 298→598→1998 IM5→IM6 −69.16→−68.94→−69.36 298→548→1998 IM6→P2 52.29→54.08→44.51 298→598→1998 HCN+H2O→NH3+CO −783.63→−784.19→−780.86 298→748→1998 表 6 HCN与H2/H2O热力学可行反应的熵变△S
Table 6 △S of thermodynamically feasible reactions between HCN and H2/H2O
Reaction △S/(J·mol−1·K−1) T/K R1→IM1 −104.37→−124.84→123.16 298→1198→1998 IM1→IM2 −117.06→−128.18→124.18 298→948→1998 IM2→P1 −19.06→−8.06 298→1998 HCN+3H2→CH4+NH3 −240.49→−266.16→−255.4 298→948→1998 R2→IM5 −136.74→−143.44→−133.73 298→648→1998 IM5→IM6 4.84→5.49→5.05 298→548→1998 IM6→P2 52.47→57.25→49.9 298→598→1998 HCN+H2O→NH3+CO −79.42→−80.94→−78.78 298→748→1998 表 7 HCN与H2/H2O各步反应速率常数
Table 7 Reaction rate constant of HCN and H2/H2O in Each Step
Reaction k/(s−1·M−1) 298 K 673 K 1073 K 1473 K 1873 K R(HCN+3H2)→IM1 1.03×10−53 6.05×10−19 1.42×10−8 9.57×10−4 6.45×10−1 IM1→IM2 4.31×10−48 4.93×10−17 1.02×10−7 2.37×10−3 8.89×10−1 IM2→P1 4.08×10−56 9.26×10−20 9.38×10−9 1.32×10−3 1.37 R(HCN+H2O)→IM5 6.67×10−34 7.17×10−11 7.06×10−4 1.71 1.70×102 IM5→IM6 1.45×10−11 2.77×102 2.60×106 1.82×108 2.16×109 IM6→P4 3.24×10−44 4.24×10−12 1.90×10−2 5.40×102 2.00×105 表 8 HCN与H2/H2O各步反应的两参数阿伦尼乌斯方程
Table 8 The two parameter Arrhenius Equation for the reaction of HCN with H2/H2O in each step
Reaction A/s−1 Ea /( kJ·mol−1) Arrhenius equation R2 R(HCN+3H2)→IM1 4.45×109 357.62 k=4.46×109 e−43 011.61/T 0.99997 IM1→IM2 5.09×108 320.28 k=5.09×108 e−38 520.66/T 0.99994 IM2→P1 2.48×1010 375.89 k=2.48×1010 e−45 208.97/T 0.99991 R(HCN+H2O)→IM5 4.68×108 239.30 k=4.68×108 e−28781.05/T 0.99977 IM5→IM6 1.25×1013 136.68 k=1.25×1013 e−16679.3/T 0.99997 IM6→P4 2.67×1014 330.70 k=2.67×1014 e−39773.89/T 0.99997 -
[1] AXEL S, JONAS S. Main group cyanides: from hydrogen cyanide to cyanido-complexes[J]. Rev Inorg Chem,2023,43:49−188. doi: 10.1515/revic-2021-0044 [2] GB 16297—1996, 大气污染物综合排放标准[S].GB 16297—1996, Comprehensive Emission Standards for Air Pollutants[S].) [3] GB 31571—2015, 石油化学工业污染物排放标准[S].GB 31571—2015, Emission Standards for Pollutants in the Petrochemical Industry[S].) [4] 伊志豪, 孙杰, 李吉刚, 等. 气相中氰化氢消除研究进展[J]. 精细化工,2021,38(1):62−70.YI Zhihao, SUN Jie, LI Jigang, et al. Research progress on the elimination of hydrogen cyanide in gas phase[J]. Fine Chem,2021,38(1):62−70. [5] 陈平平, 胡德豪. 重油气化装置含氰废水处理技术对比[J]. 石油炼制与化工,2020,51(8):98−103.CHEN Pingping, HU Dehao. Comparison of treatment technologies for cyanide containing wastewater from heavy oil gasification plants[J]. Pet Ref Chem Ind,2020,51(8):98−103. [6] 张奉民, 李开喜, 吕春祥, 等. 氰化氢脱除方法[J]. 新型炭材料,2003,02:151−157. doi: 10.3321/j.issn:1007-8827.2003.02.014ZHANG Fengmin, LI Kaixi, LV Chunxiang, et al. Hydrogen cyanide removal method[J]. New Carbon Mater,2003, 18 (2):151−157. doi: 10.3321/j.issn:1007-8827.2003.02.014 [7] OLIVER T, JUGOSLAV K, ALEKSANDAR P, et al. Synthetic activated carbons for the removal of hydrogen cyanide from air[J]. Chem Eng Process,2005,44(11):1181−1187. doi: 10.1016/j.cep.2005.03.003 [8] TAN H, WANG X, WANG C, et al. Characteristics of HCN removal using CaO at high temperatures[J]. Energy Fuels,2009,23(2):1545−1550. [9] PATERSON N, ZHOU Y, DUGWELL D, et al. Formation of hydrogen cyanide and ammonia during the gasification of sewage sludge and bituminous coal[J]. Energy Fuels,2005,19(3):1016−1022. doi: 10.1021/ef049688h [10] SCHAFER S, BONN B. Hydrolysis of HCN as an important step in nitrogen oxide formation in fluidized combus-tion. Part 1. Homogeneous reactions[J]. Fuel,2000,79(10):1239−1246. doi: 10.1016/S0016-2361(99)00254-9 [11] FRIEBEL R F W K. The fate of nitrogen during pyrolysis of German low rank coals-a parameter study[J]. Fuel,1999,78(8):923−932. doi: 10.1016/S0016-2361(99)00008-3 [12] 彭国建, 杨蒙, 王国微, 等. HCN消除反应机理的理论研究[J]. 分子催化,2022,36(5):446−455.PENG Guojian, YANG Meng, WANG Guowei, et al. Theoretical study on the mechanism of HCN elimination reaction[J]. J. Mol. Catal. (China),2022,36(5):446−455. [13] 侯封校, 金晶, 王永贞, 等. 污泥热解中HCN与CaO的反应机理: 密度泛函理论研究[J]. 燃料化学学报,2017,45(1):123−128.HOU Fengxiao, JIN Jing, WANG Yongzhen, et al. Reaction mechanism of HCN and CaO in sludge pyrolysis: density functional theory study[J]. J Fuel Chem Technol,2017,45(1):123−128. [14] DARLA N, SHARMA D, SITHA S. Formation of formamide from HCN+H2O: A computational study on the roles of a second H2O as a catalyst, as a spectator, and as a reactant[J]. J Phys Chem A,2020,124(1):165−175. doi: 10.1021/acs.jpca.9b09924 [15] 付蓉, 卢天, 陈飞武. 亲电取代反应中活性位点预测方法的比较[J]. 物理化学学报,2014,30(4):628−639. doi: 10.3866/PKU.WHXB201401211FU Rong, LU Tian, CHEN Feiwu. Comparison of active site prediction methods in electrophilic substitution reactions[J]. J Phys Chem,2014,30(4):628−639. doi: 10.3866/PKU.WHXB201401211 [16] OLAH J, Van A , Sannigrahi A. condensed fukui functions derived from stockholder charges: Assessment of their performance as local reactivity descriptors[J]. J Phys Chem A,2002,106(15):3885−3890. [17] LU T, CHEN F. Multiwfn: A multifunctional wavefunction analyzer[J]. J Comput Chem,2012,33:580−592. doi: 10.1002/jcc.22885 [18] :于遵宏, 王辅臣, 等. 煤炭气化技术[M]. 北京 化学工业出版社, 2010.YU Zunhong, WANG Fuchen, et al. Coal Gasification Technology[M]. Beijing: Chemical Industry Press, 2010.) [19] FRISCH M, TRUCKS G, SCHLEGEL H, et al. Gaussian 09, Revision A. 02[CP]. Wallingford, CT: Gaussian, Inc. 2016. [20] CHAI D, HEAD-GORDON M. Long-range corrected hybrid density functionals with damped atom-atom disperse-on corrections[J]. Phys Chem Chem Phys,2008,10(44):6615−6620. doi: 10.1039/b810189b [21] MATITO E, POATER J, MIQUEL S, et al. Comparison of the AIM delocalization index and the Mayer and fuzzy atom bond orders.[J]. J Phys Chem A,2005,109(43):9904−9910. doi: 10.1021/jp0538464 [22] LU T, CHEN Q. Shermo: A general code for calculating molecular thermochemistry properties[J]. Comput Theor Chem,2021,1200:113249. doi: 10.1016/j.comptc.2021.113249 [23] MERRICK J , MORAN D, RADOM L. An evaluation of harmonic vibrational frequency scale factors[J]. J Phys Chem A,2007,111(45):11683−11700. [24] HAMID A, ROY R. Correlation between equilibrium constant and stabilization energy: A combined approach based on chemical thermodynamics, statistical thermodynamics, and density functional reactivity theory[J]. J Phys Chem A,2020,124(7):1279−1288. doi: 10.1021/acs.jpca.9b07920 [25] SEBASTIEN C, FREDERIC B, ERIC H. KiSThelP: A program to predict thermodynamic properties and rate constants from quantum chemistry results[J]. J Comput Chem,2013,35(1):82−93. [26] BARADYN M, RATKIEWICZ A. On-the-fly kinetics of the hydrogen abstraction by hydroperoxyl radical: An application of the reaction class transition state theory[J]. Front Chem,2021,9:806−873. [27] WIGNER P. On the quantum correction for thermodynamic equilibrium[J]. Phys Rev,1997,40:749−759. [28] BRENNAN K, WASTON W, GARCIA-MELCHOR M. A computational study of the electrochemical cyanide reduction for ambient ammonia production on a nickel cathode[J]. Catal Sci Technol,2021,16:5633−5640. [29] CAROLINA O, VAN D , DANIEL C, et al. A density functional theory study of HCN hydrogenation to methylamine on Ni(111)[J]. J Catal,2007,245:436−445. [30] TANNOUS J H , KLERK A. Methyl and Hydrogen Transfer in Free Radical Reactions[J]. Energy Fuels,2020,34(02):1698−1709. [31] 天津大学无机化学教研室. 无机化学[M]. 北京: 高等教育出版社, 2018.Inorganic Chemistry Teaching and Research Office of Tianjin University. Inorganic Chemistry[M]. Beijing: Higher Education Press, 2018.) [32] KROCHER O, ELSENER M. Hydrolysis and oxidation of gaseous HCN over heterogeneous catalysts[J]. Appl Catal B: Environ,2009,92(1/2):75−89. doi: 10.1016/j.apcatb.2009.07.021 [33] KUA J, THRUSH L. HCN, Formamidic acid and formamide in aqueous solution: A free energy map.[J]. J Phys Chem B,2016,120(33):8175−8185. doi: 10.1021/acs.jpcb.6b01690 [34] 彭昌军, 胡英. 物理化学[M]. 北京: 高等教育出版社 2021.PENG Changjun, HU Ying. Physical Chemistry[M]. Beijing: Higher Education Press, 2021.) [35] YACAI H, QI C, YAVUAN H, et al. The generalized thermodynamic temperature and the new expressions of the first and the second law of thermodynamics[J]. J Therm Sci,2016,25:1−6. doi: 10.1007/s11630-016-0827-1 [36] JOSEPH M. What is the rate-limiting step of a multistep reaction[J]? J Chem Educ,1981,58(1):32−38. doi: 10.1021/ed058p32 -





 下载:
下载: