Density functional theory study of adsorption of As2O3 on CeO2 surface by Fe, La doping and oxygen defects
-
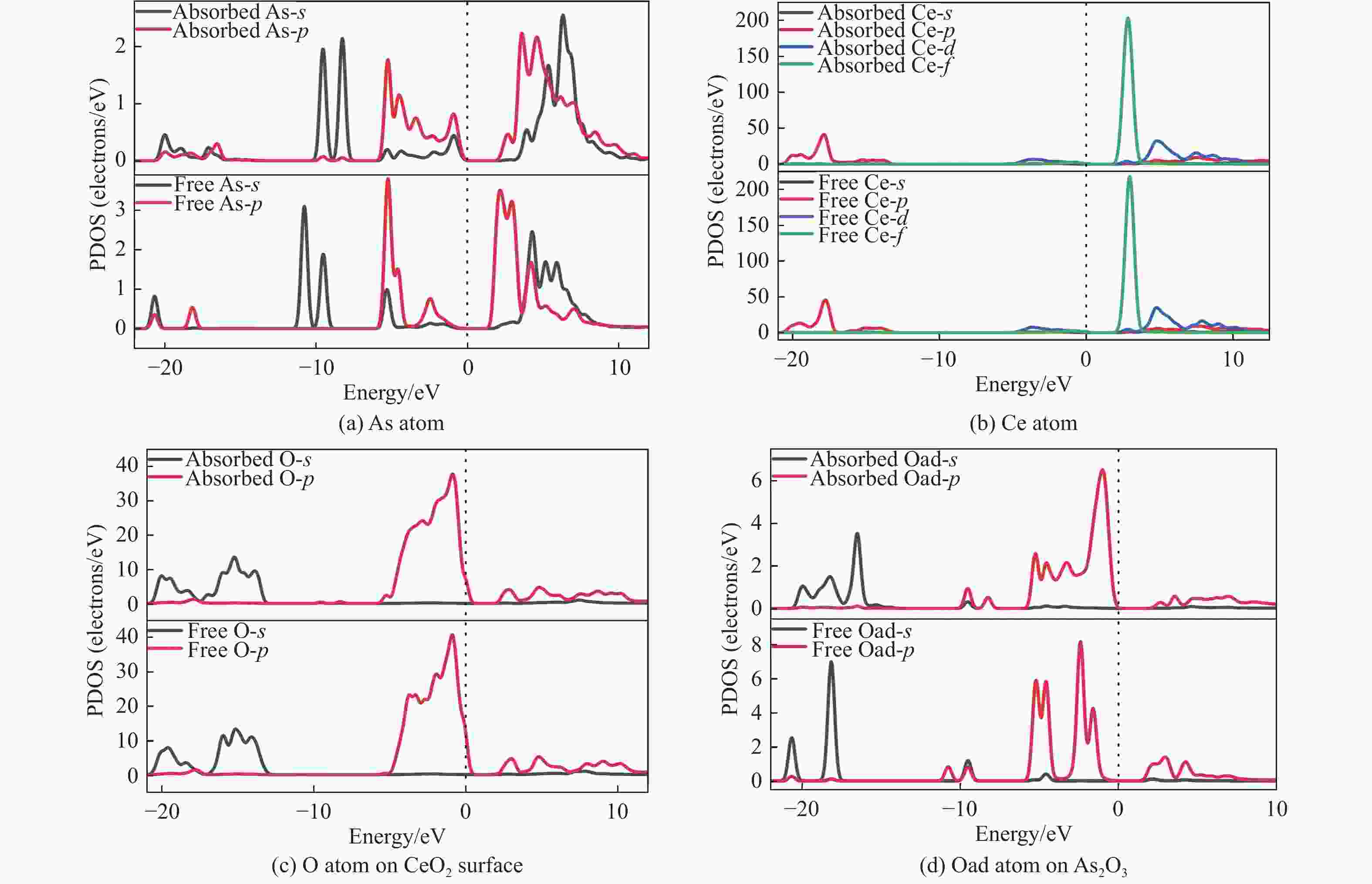
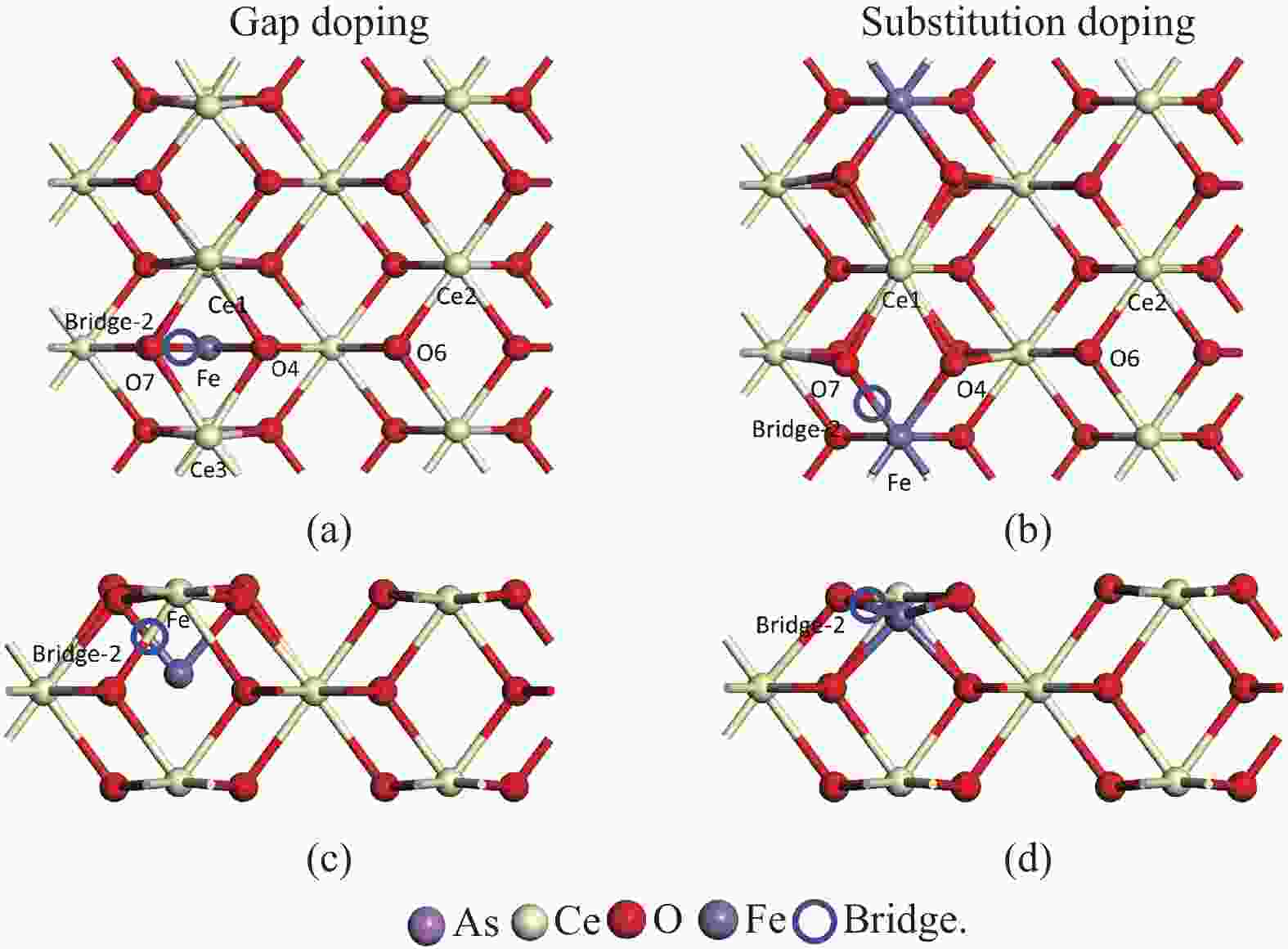
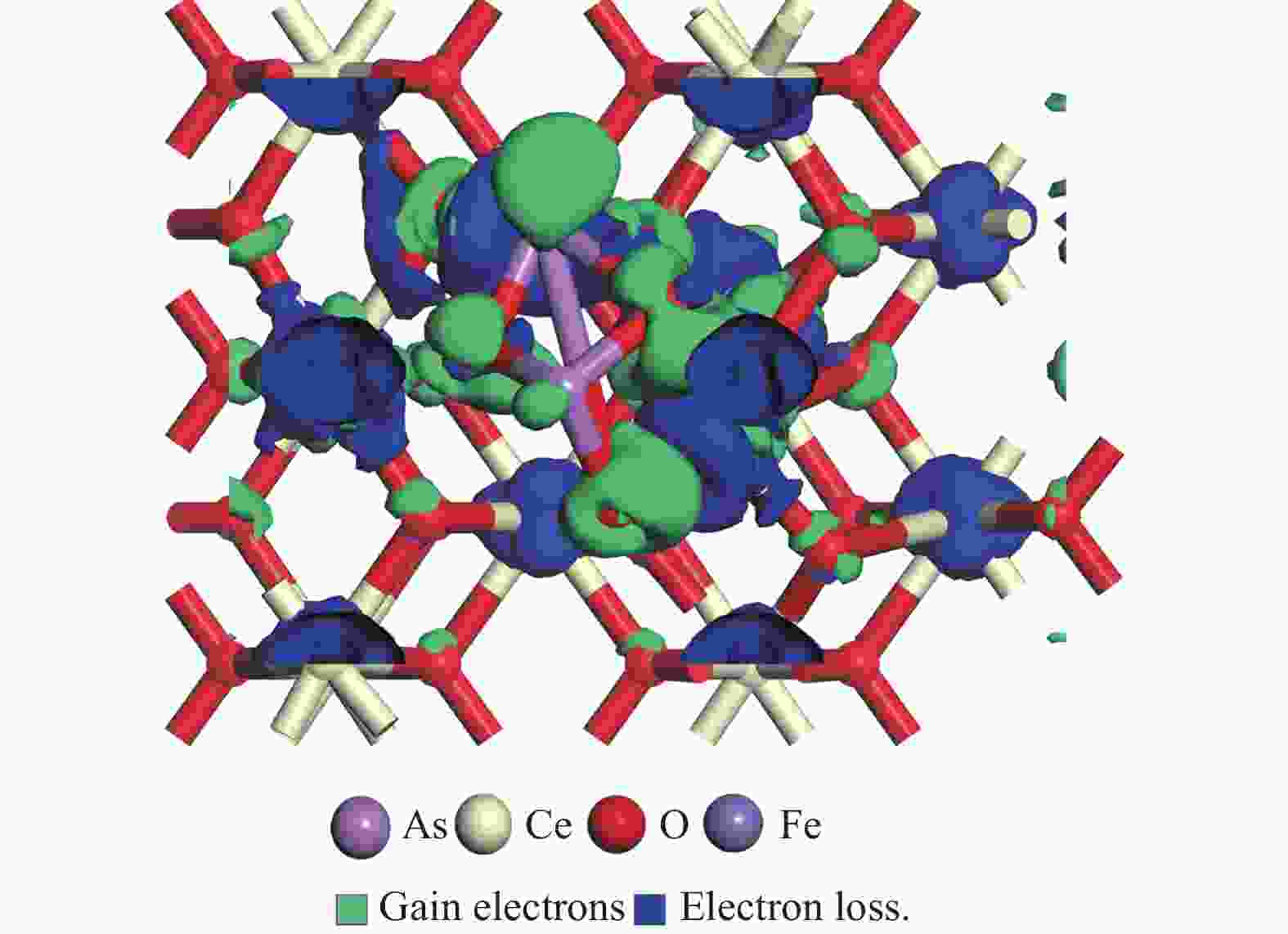
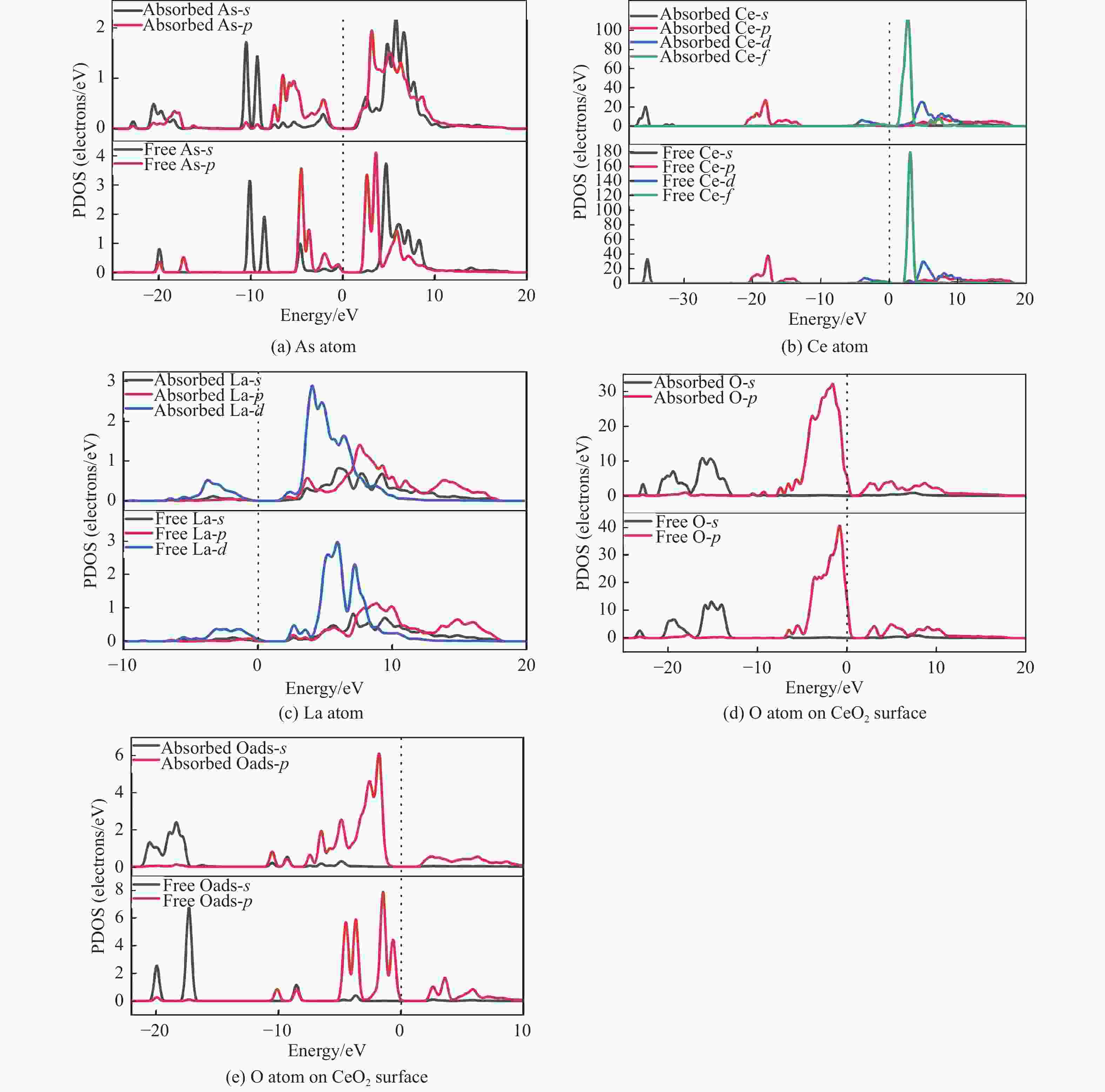
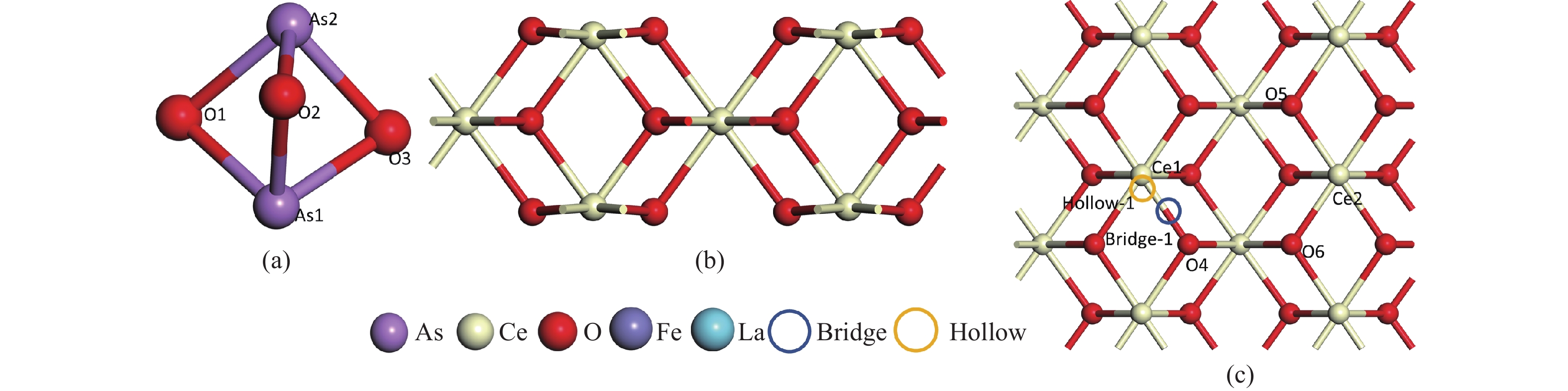
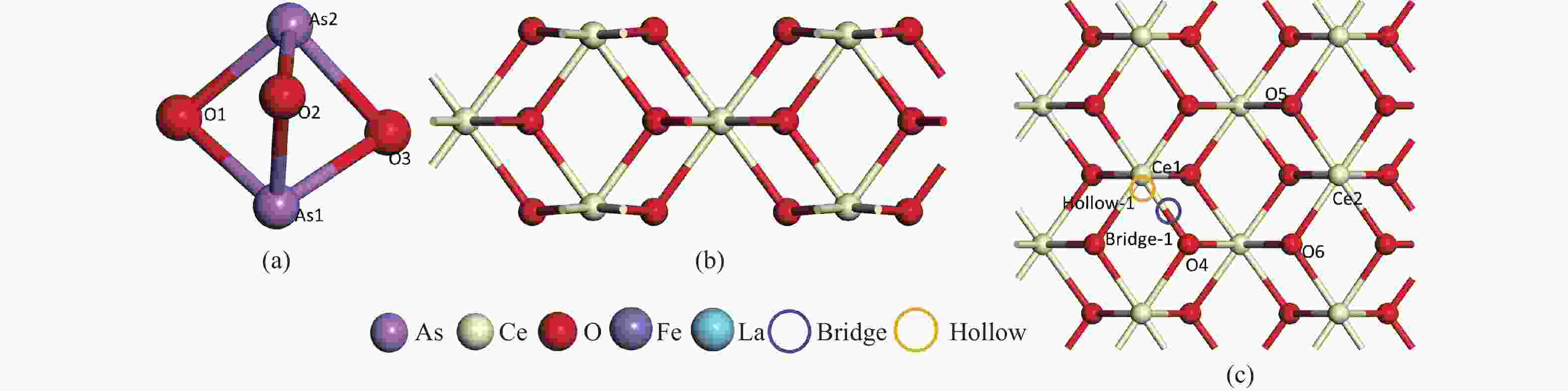
摘要: 采用密度泛函理论研究了As2O3(g)在Fe、La掺杂CeO2(110)表面及氧缺陷LaCeO(110)表面的吸附行为,探索了LaCeO表面砷吸附能力显著高于FeCeO表面的主要原因。结果表明,As2O3(g)的吸附效果与吸附位点数量、吸附能、键长和电荷转移密切相关。纯CeO2表面的吸附主要为化学吸附,吸附能绝对值大于−4.22 eV,电荷转移量为−0.19− −0.31 e,As2O3得到电荷带负电,起表面受主作用,因此吸附量较小。FeCeO(110)表面新增Fe顶位和Bridge-2桥位两个吸附位,其中,Fe顶位为化学吸附,Fe掺杂改变了FeCeO表面电子分布和晶格结构,但并未改变As2O3与FeCeO之间的电荷转移方向,因此,As2O3仍呈负离子形式吸附。LaCeO(110)表面新增了三个吸附位:La顶位、Bridge-3桥位和Hollow-2空位,La掺杂改变了As2O3与LaCeO之间的电荷转移方向,使得As2O3失电子呈正离子吸附,起表面施主作用,因此,吸附能力增强。无O2环境下,单一O缺陷LaCeO(110)表面吸附能力低于完整LaCeO表面;有O2环境下,O缺陷有利于As2O3的吸附。Abstract: Density functional theory (DFT) was used to study the adsorption behavior of As2O3 (g) on iron and lanthanum doped CeO2 (110) and oxygen-deficient LaCeO (110) surfaces, and the reasons for the arsenic adsorption capacity of LaCeO surface was significantly higher than that of FeCeO surface was explored. The results show that the adsorption effect of As2O3 (g) is closely related to the number of adsorption sites, adsorption energy, bond length and charge transfer amount. Ce and O atoms on the surface of pure CeO2 are both active sites, and the adsorption is mainly chemisorption, the absolute adsorption energy is greater than −4.22 eV, and the charge transfer amount is −0.19− −0.31 e. As2O3 has a negative charge and acts as a surface accepter, while CeO2 loses electrons and has a positive charge on the surface, which acts as a surface donor. The number of free electrons in the CeO2 conduction band gradually decreases, the conductivity decreases, and it is difficult to provide more electrons continuously, so the adsorption amount is small. Two adsorption sites are added on the surface of FeCeO (110): Fe top site and Bridge-2 Bridge site, where Fe top site is chemical adsorption and Bridge-2 Bridge site is physical adsorption. The gap doping of Fe changes the electron distribution and lattice structure on the surface of FeCeO, resulting in obvious deformation of the lattice and reducing the difficulty of bonding, thus increasing the configurational adsorption energy of some configurations. However, it does not change the charge transfer direction between As2O3 and FeCeO, thus not changing the surface adsorption form of As2O3. As2O3 is still adsorbed in the form of negative ions, which plays the role of surface acceptor, and the adsorption amount is small. LaCeO (110) has three new adsorption sites: La top site, Bridge-3 Bridge site and Hollow-2 vacancy, among which the La top site and Bridge-3 Bridge site are chemical adsorption. La doping changes the charge transfer direction between As2O3 and LaCeO, resulting in positive ion adsorption of As2O3 with electron loss and surface donor function. The electrons on the surface of LaCeO play the role of surface acceptor. With the progress of adsorption, the number of free electrons in the conduction band increases, and the conductivity increases. Therefore, the adsorption capacity of As2O3 on the surface of LaCeO increases. In the absence of O2, the number of chemical bonds and bond energy formed on the surface of LaCeO (110) with single O defect are smaller than those on the surface of LaCeO, and the charge transfer on the surface of the defect is less, so the adsorption energy decreases. In this case, As2O3 obtains electrons and acts as the surface donor, and the adsorption capacity is lower than that on the complete LaCeO surface. In the presence of O2, the adsorption energy and charge transfer number increase in the ortho-configuration after O2 supplementation with O defect. As2O3 is positively adsorbed in ionic form, and the adsorption energy is also higher than that on the intact LaCeO surface. The adsorption capacity of As2O3 is better than that on the LaCeO surface, indicating that O defect is conducive to the adsorption of As2O3 in the presence of O2.
-
Key words:
- density functional theory /
- CeO2 /
- Fe、La doping /
- As2O3 adsorption /
- oxygen deficiency
-
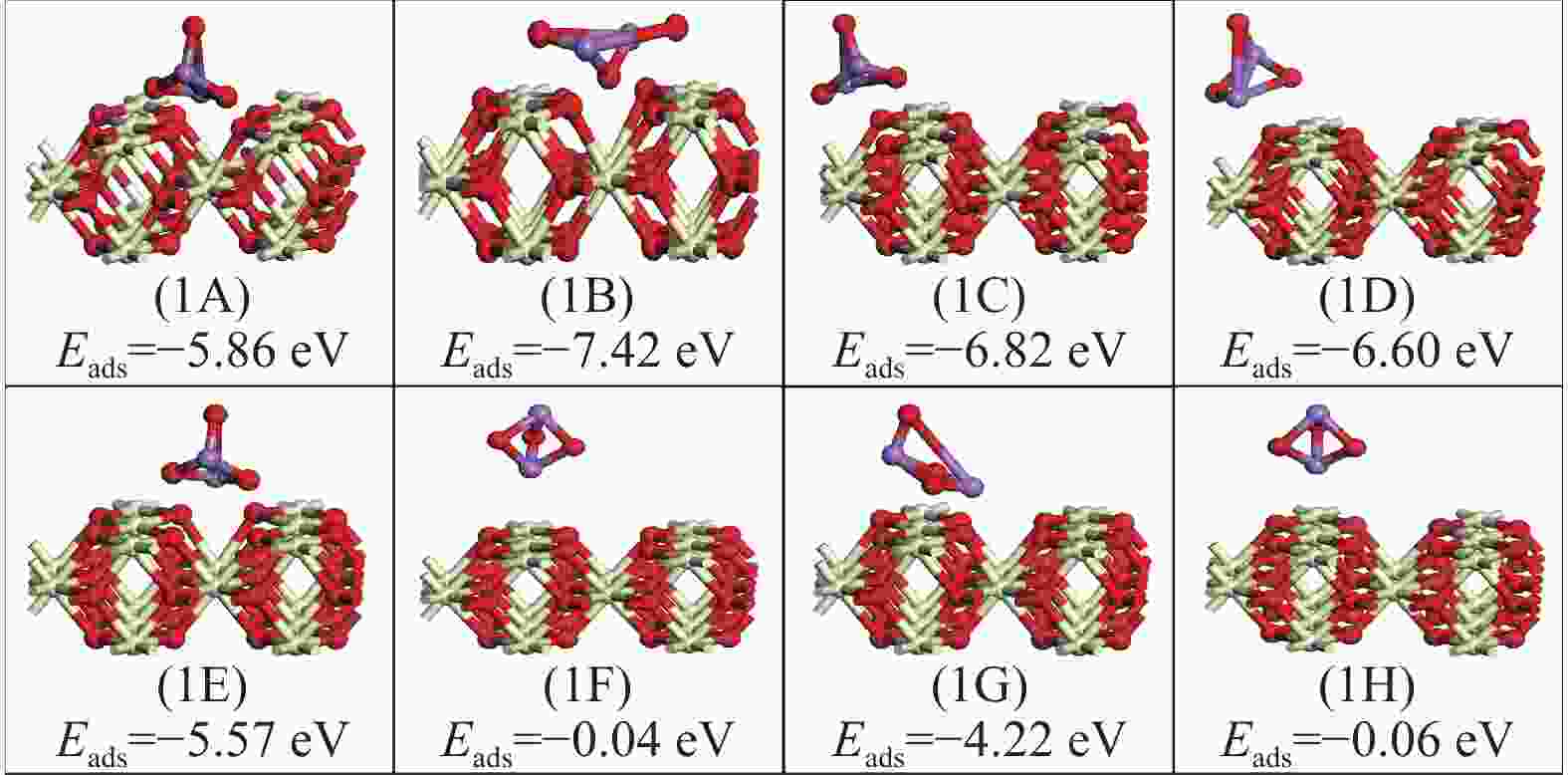
表 1 As2O3分子在CeO2(110)表面的吸附能、键长和电荷转移
Table 1 Adsorption energy, bond length and charge transfer of As2O3 molecules on CeO2 (110) surface
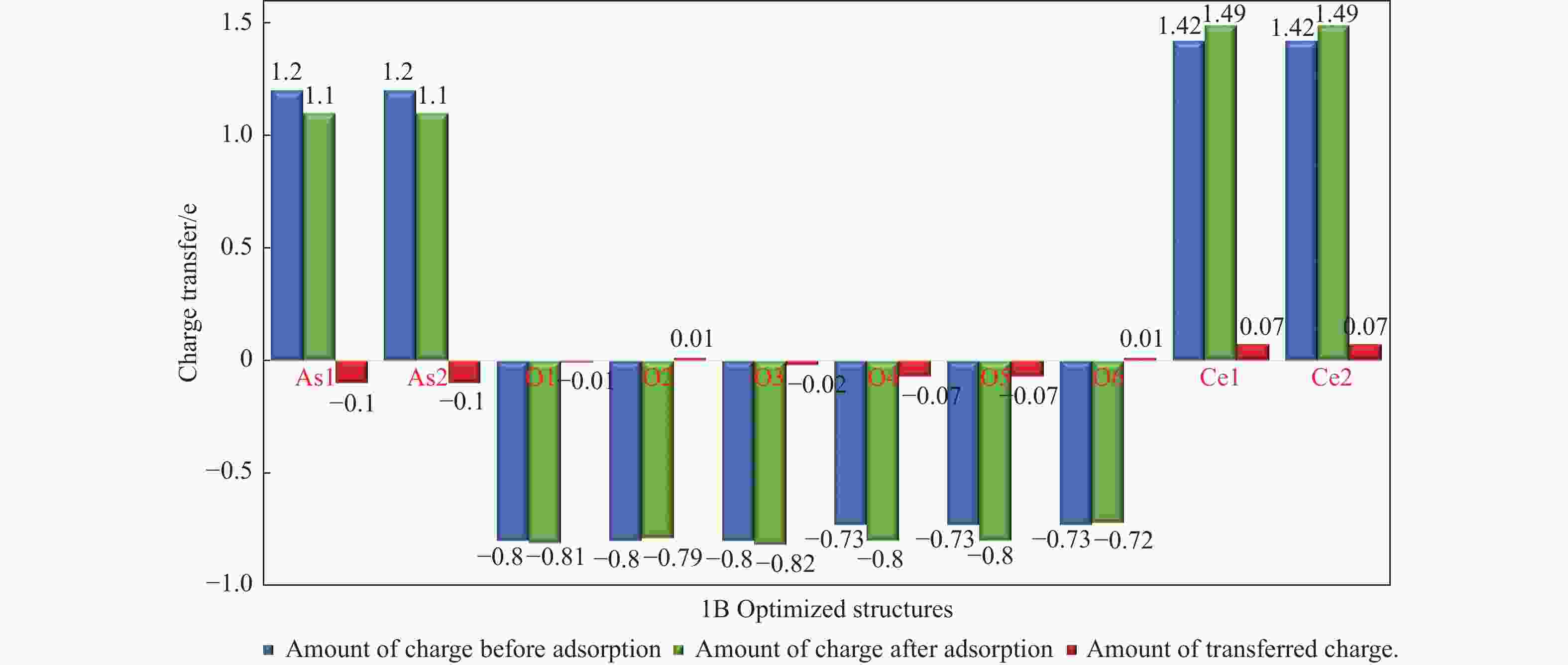
Adsorption structure(X-Y) Ead/eV RAs-O/Å RCe-Oads/Å ΔQ/e 1A O2-O5 −5.89 1.90 2.33 −0.28 1B As1-O5 −7.42 1.84 2.31 −0.22 1C O2-Ce1 −6.82 1.87 2.26 −0.29 1D As1-Ce1 −6.60 1,87 2.24 −0.28 1F 02-Bridge-1 −5.57 1,92 2.35 −0.31 1E As1-Bridge-1 −0.04 4.41 4.97 −0.04 1G 02-Hollow1 −4.22 1.82 2.38 −0.19 1H As1-Hollow1 −0.06 4.41 4.39 −0.06 表 2 As2O3分子在FeCeO(110)表面的吸附能、键长和电荷转移
Table 2 Adsorption energy, bond length and charge transfer of As2O3 molecules on FeCeO (110) surface
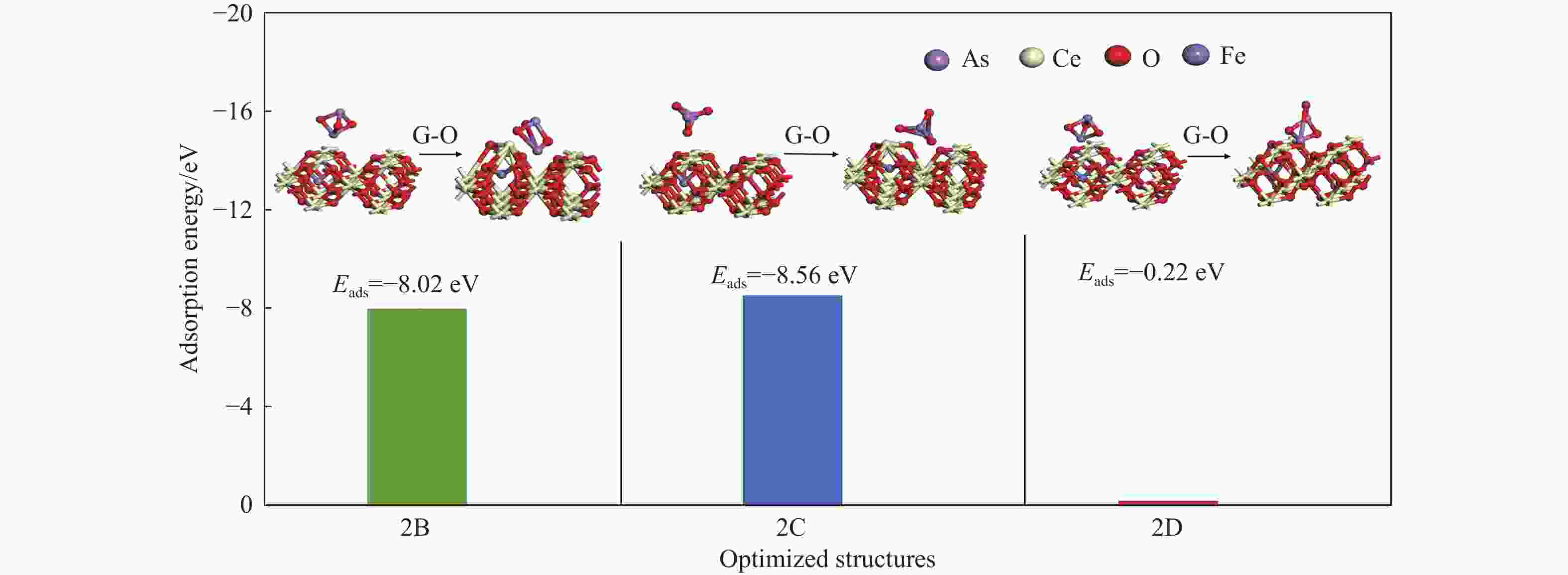
Adsorption structure(X-Y) Ead/eV RAs-O/Å RCe-Oads/Å ΔQ/e 2A O2-O5 −6.55 1.85 4.31 −0.20 2B As1-O5 −8.02 1.84 2.27 −0.14 2C O2-Ce1 −8.56 1.82 2.06 −0.22 2D As1-Ce1 −0.22 4.03 4.58 −0.05 2E O2-Fe −2.54 1.82 2.45 −0.2 2F As1-Fe −4.42 1.93 2.29 −0.32 2G O2- Bridge-1 −8.29 1.82 2.27 −0.23 2H As1- Bridge-1 1.51 4.08 4.78 −0.06 2I O2-Bridge-2 0.26 2.98 4.42 −0.05 2J As- Bridge-2 0.31 2.95 2.51 −0.07 表 3 As2O3分子在LaCeO(110)表面的吸附能、键长和电荷转移
Table 3 Adsorption energy, bond length and charge transfer of As2O3 molecules on LaCeO (110) surface
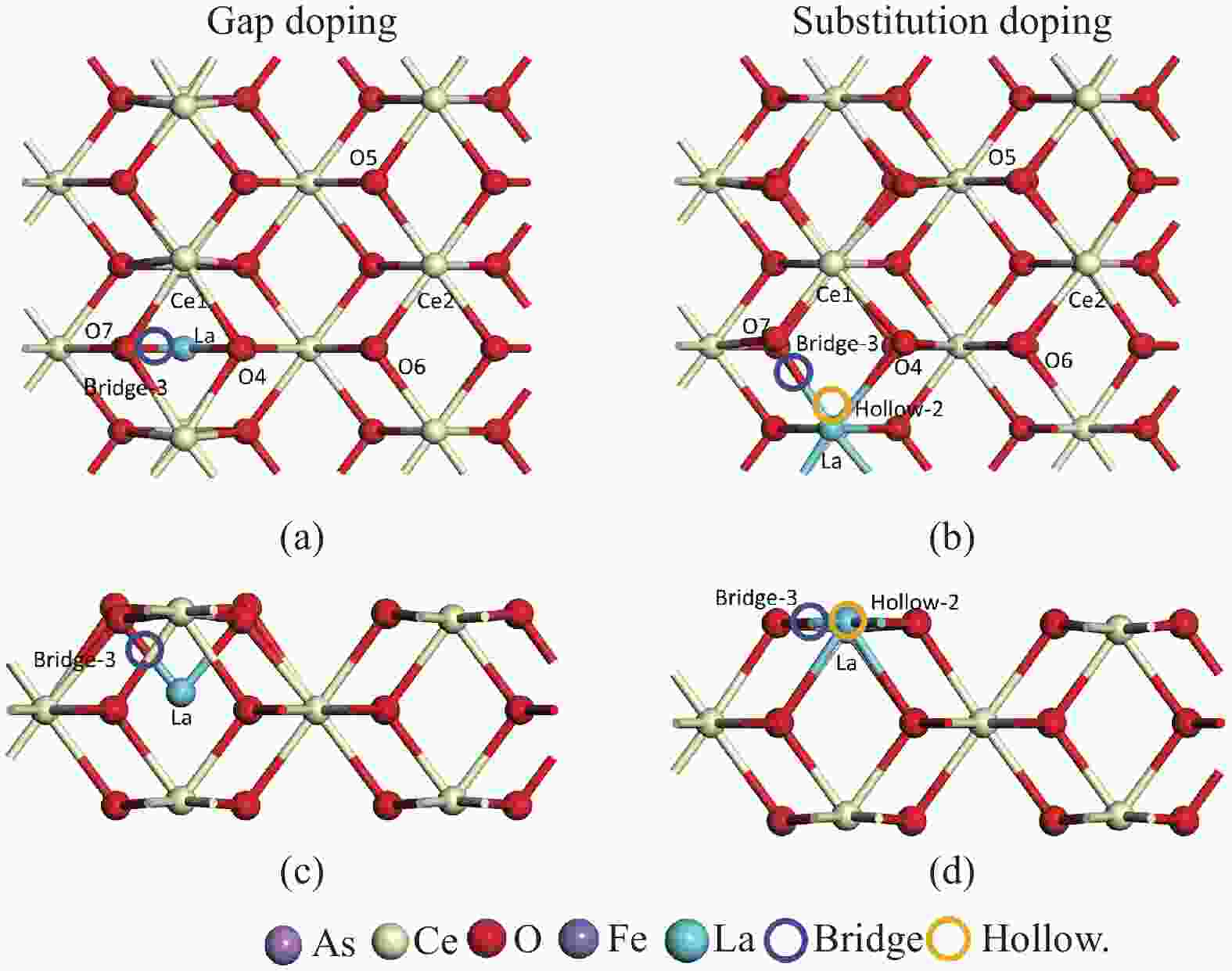
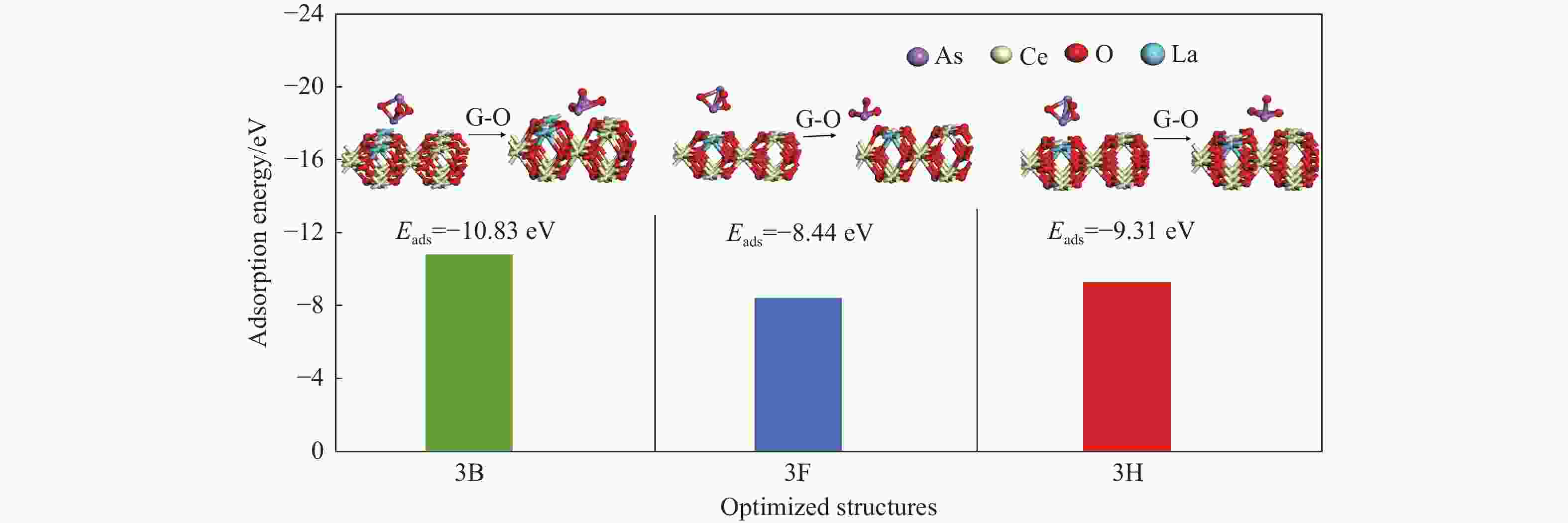
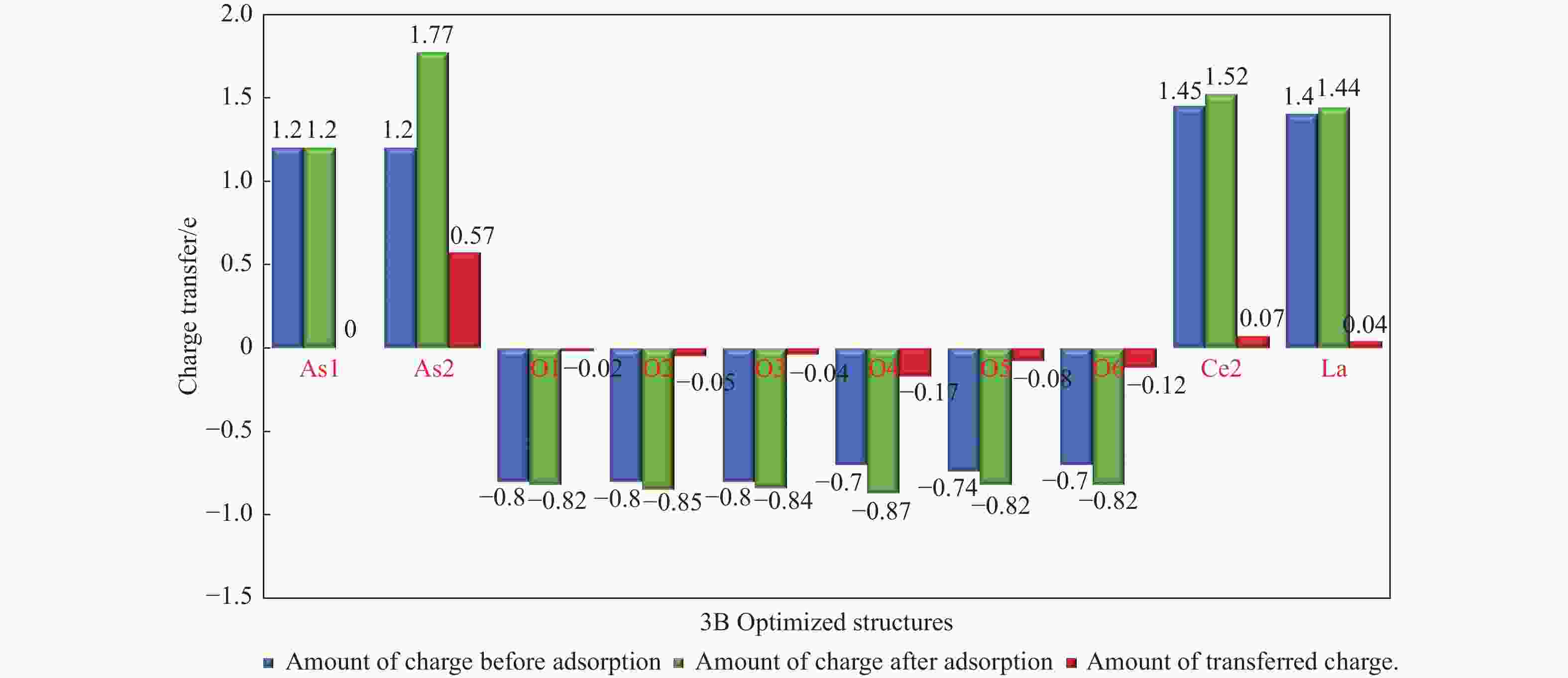
Adsorption structure (X-Y) Ead/eV RAs-O/Å RCe-Oads/Å ΔQ/e 3A O2-O5 −7.74 1.91 2.36 0.36 3B As1-O5 −10.83 1.78 2.42 0.46 3C O2-Ce1 −11.09 1.78 2.51 0.45 3D As1-Ce1 −10.13 1.91 4.76 0.35 3E O2-La −0.81 2.66 6.12 −0.04 3F As1-La −8.44 1.89 2.52 0.37 3G O2- Bridge-1 −8.56 1.88 2.43 0.39 3H As1- Bridge-1 −9.31 1.91 2.36 0.35 3I O2- Bridge-3 −12.53 1.79 2.60 0.37 3J As1- Bridge-3 −10.70 1.88 2.43 0.36 3K 02-Hollow-1 −11.81 1.79 2.51 0.46 3L As1-Hollow-1 −11.82 1.81 2.51 0.43 3M 02-Hollow-2 −10.29 1.91 3.87 0.36 3N As1-Hollow-2 −0.08 4.20 5.12 −0.04 表 4 As2O3分子在LaCeO(Ov)(110)表面的吸附能、键长和电荷转移
Table 4 Adsorption energy, bond length and charge transfer of As2O3 molecules on LaCeO(Ov) (110) surface
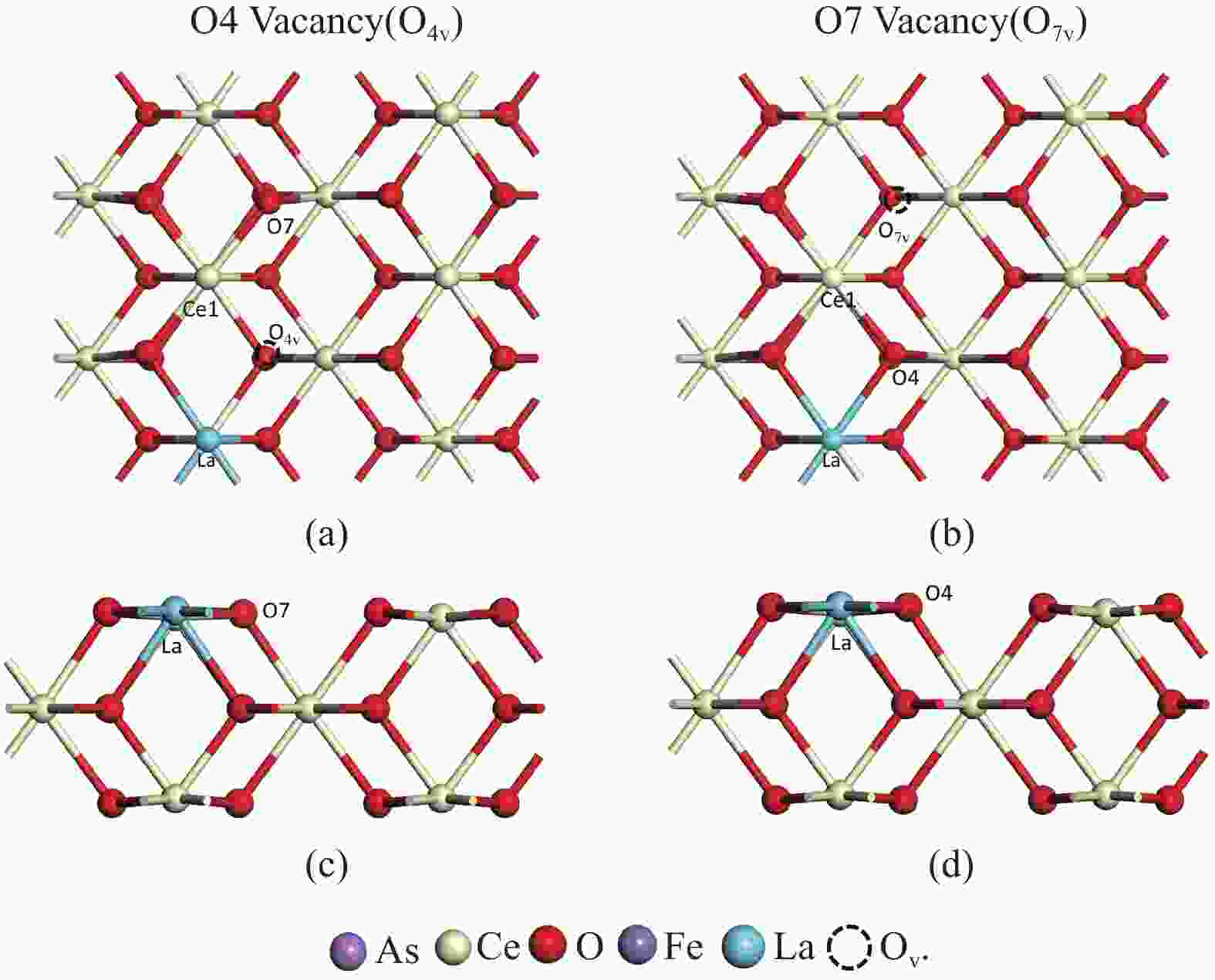
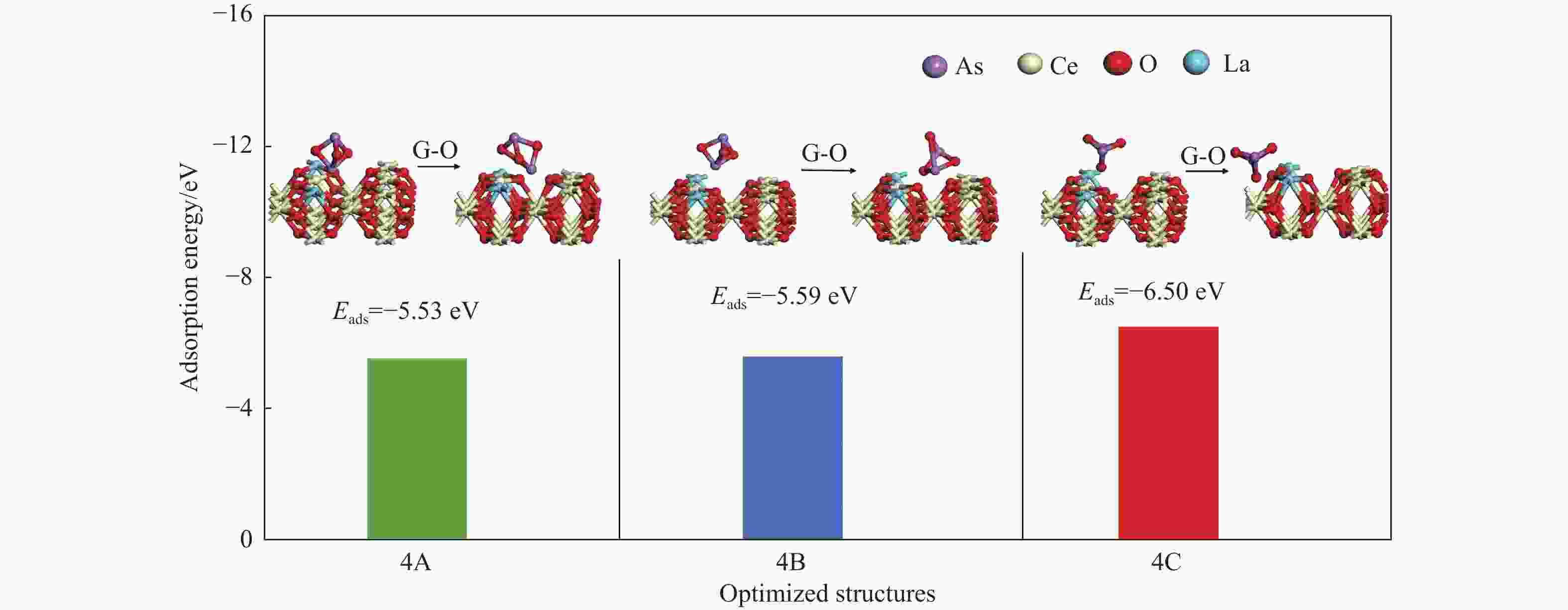
Adsorption structure Ead RAs-O/Å RCe-Oads/Å ΔQ/e 4A As1-O4v −5.53 1.76 2.50 −0.12 4B As1-O4(O7v) −5.59 1.86 2.36 −0.24 4C O2−O4(O7v) −6.50 1.98 4.85 −0.30 表 5 As2O3分子在LaCeO(O2)(110)表面的吸附能、键长和电荷转移
Table 5 Adsorption energy, bond length and charge transfer of As2O3 molecules on LaCeO(O2) (110) surface
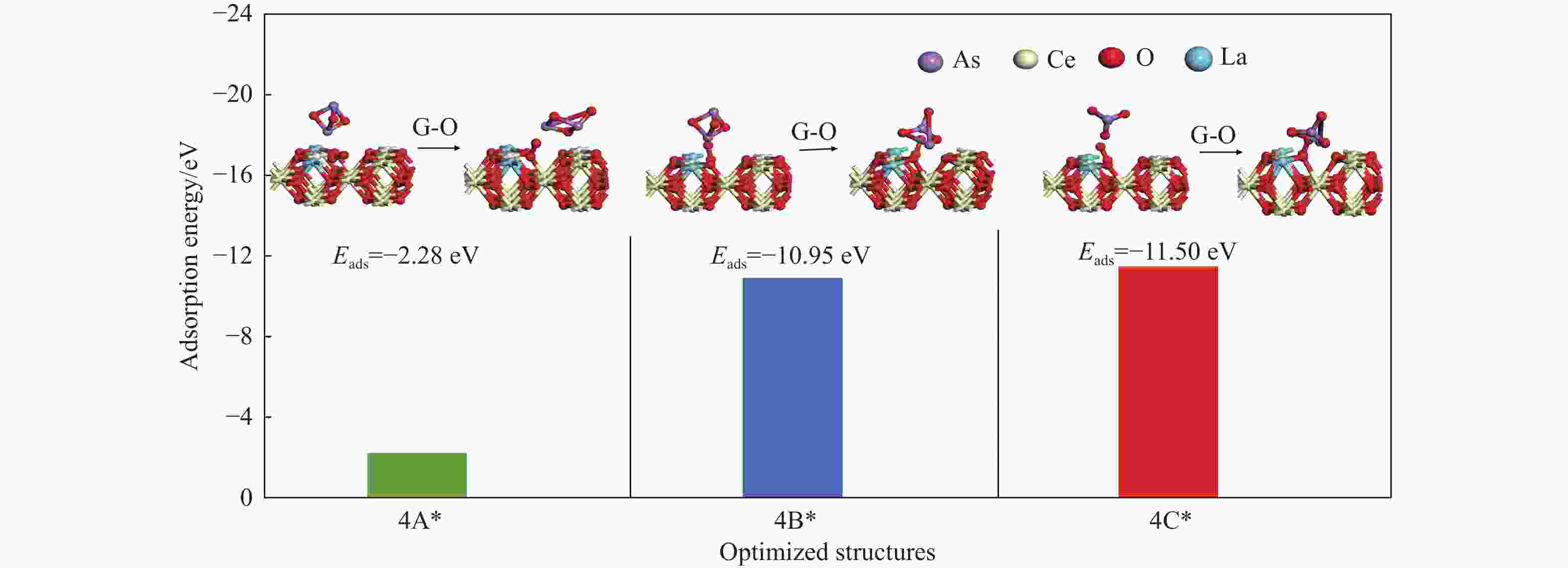
Adsorption structure Ead RAs-O/Å RCe-Oads/Å ΔQ/e 4A* As1- O4v(O2) −2.28 1.76 2.50 −0.25 4B* As1-O4(O7v-O2) −10.95 1.80 2.47 0.52 4C* O2-O4(O7v-O2) −11.50 1.78 2.91 0.44 -
[1] 刘明亮, 卫浩, 盖玉龙, 等. 中国、美国、欧盟及世界一次能源消费现状与展望[J]. 煤化工, 2022, 50(02): 1-5.LIU Mingliang, WEI Hao , GAI Yulong, et al. Current situation and outlook of primary energy consumption in China, US, EU and the world [J]. Coal Chem. Ind, 2022, 50(02): 1-5.) [2] TIAN H Z, WANG Y, XUE Z G, et al. Trend and characteristics of atmospheric emissions of Hg, As, and Se from coal combustion in china, 1980–2007[J]. Atmos. Chem. Phy,2010,10(23):11905−11919. doi: 10.5194/acp-10-11905-2010 [3] TANG Q, LIU G, YAN Z, et al. Distribution and fate of environmentally sensitive elements (arsenic, mercury, stibium and selenium) in coal-fired power plants at huainan, anhui, china[J]. Fuel,2012,95:334−339. doi: 10.1016/j.fuel.2011.12.052 [4] QUISPE D, PÉREZ-LÓPEZ R, SILVA L F O, et al. Changes in mobility of hazardous elements during coal combustion in santa catarina power plant (brazil)[J]. Fuel,2012,94:495−503. doi: 10.1016/j.fuel.2011.09.034 [5] XUE Y, WANG Y. Effective industrial regeneration of arsenic poisoning waste selective catalytic reduction catalyst: contaminants removal and activity recovery[J]. Environ. Sci. Pollut. Res,2018,25(34):34114−34122. doi: 10.1007/s11356-018-3369-0 [6] CIMINO S, LISI L. Catalyst deactivation, poisoning and regeneration[J]. Catalysts,2019,9(8):668. doi: 10.3390/catal9080668 [7] 王俊杰, 张亚平, 李娟, 等. 砷中毒商业V2O5-WO3/TiO2催化剂的再生方法[J]. 工程热物理学报,2018,39(2):450−456.WANG Junjie, ZHANG YaPing, LI Juan, et al. Regeneration Method of arsenic poisoned commerical SCR Catalyst[J]. J. Eng. Thermophys,2018,39(2):450−456. [8] HU P, WANG S, ZHUO Y. Research on As2O3 adsorption enhancement characteristics of Mn-modified γ-Al2O3[J]. Chem. Eng. J,2021,426:131660. doi: 10.1016/j.cej.2021.131660 [9] 曹蕃, 苏胜, 向军, 等. Mn-Ce-Zr/γ-Al2O3催化剂低温选择性催化还原脱硝性能分析[J]. 中国电机工程学报,2015,35(9):2238.CAO Fan, SU Sheng, XIANG Jun, et al. Performances of Mn-Ce-Zr/γ-Al2O3 Catalyst for Low Temperature Selective Catalytic Reduction of NO[J]. CSEE JPES,2015,35(9):2238. [10] ZHANG Y, LIU J. Density functional theory study of arsenic adsorption on the Fe2O3(001) surface[J]. Energy Fuels,2019,33(2):1414−1421. doi: 10.1021/acs.energyfuels.8b04155 [11] HU H Y, CHEN D K, LIU H, et al. Adsorption and reaction mechanism of arsenic vapors over γ-Al2O3 in the simulated flue gas containing acid gases[J]. Chemosphere,2017,180(1):186−191. [12] HWANG S, KIM Y, LEE J, et al. Promoting effect of CO on low-temperature NOx adsorption over Pd/CeO2 catalyst[J]. Catal. Today,2022,384-386:88−96. doi: 10.1016/j.cattod.2021.05.022 [13] ZHAO G, LI M, LI H, et al. La-doped micro-angular cube ZnSnO3 with nano-La2O3 decoration for enhanced ethylene glycol sensing performance at low temperature[J]. Sens. Actuators, A,2023,362:114649. doi: 10.1016/j.sna.2023.114649 [14] ZHANG K, HU L, WANG C, et al. Middle-low-temperature oxidation and adsorption of arsenic from flue gas by Fe–Ce-based composite catalyst[J]. Chemosphere,2022,288:132425. doi: 10.1016/j.chemosphere.2021.132425 [15] 侯书阳, 张凯华, 王传风, 等. Fe-Ce-La复合氧化物在中低温烟气脱砷过程中的协同作用[J]. 中国电机工程学报,2023,43(2):640−651.HOU Shuyang, ZHANG Kaihua, WANG Chuanfeng, et al. Synergistic Effect of Fe-Ce-La Composite Oxide in the Process of Arsenic Removal From Flue Gas at Middle-Low-Temperatures[J]. CSEE JPES,2023,43(2):640−651. [16] 唐楠楠, 张姝, 李强林. 密度泛函理论的基本计算方法研究进展[J]. 成都纺织高等专科学校学报, 2015, 32(2): 39-43.TANG Nannan, ZHANG Shu , LI Qianghlin. Basic Algorithms Research Progress of Density Functional Theory [J]. J. Chengdu Text. Coll, 2015, 32(2): 39-43.) [17] 马生贵, 田博文, 周雨薇, 等. 氮掺杂Stone-Wales缺陷石墨烯吸附H2S的密度泛函理论研究[J]. 化工学报,2021,72(9):4496−4503. doi: 10.11949/0438-1157.20210215MA Shenggui, TIAN Bowen, ZHOU Yuwei, et al. DFT study of adsorption of H2S on N-doped Stone-Wales defected graphene[J]. J. Chem. Eng,2021,72(9):4496−4503. doi: 10.11949/0438-1157.20210215 [18] 周文波, 牛胜利, 刘思彤, 等. γ-Fe2O3抗As2O3中毒能力的分子模拟[J]. 中国环境科学,2022,42(8):3600−3609. doi: 10.3969/j.issn.1000-6923.2022.08.014ZHOU Wenbo, NIU Shengli, LIU Sitong, et al. Molecular simulation study on the anti-As2O3 poisoning ability of γ-Fe2O3[J]. China Environ. Sci,2022,42(8):3600−3609. doi: 10.3969/j.issn.1000-6923.2022.08.014 [19] YU Y, ZHAO R, LI X, et al. Mechanism of CaO and Fe2O3 capture gaseous arsenic species in the flue gas: dft combined thermodynamic study[J]. Fuel,2022,312:122838. doi: 10.1016/j.fuel.2021.122838 [20] ZHANG Y, ZHAO B, WANG C, et al. Dual-functional effect encompassing adsorption and catalysis by mn-modified iron-based sorbents for arsenic removal: experimental and DFT study[J]. J. Hazard. Mater,2023,459:132079. doi: 10.1016/j.jhazmat.2023.132079 [21] ZHAO S, WANG Y, XIE X, et al. Arsenic removal from Coal-fired flue gas by MnO2 coated magnetic flower-like Fe3O4 composites: experimental and DFT study[J]. Chem. Eng. J,2023,478:147481. doi: 10.1016/j.cej.2023.147481 [22] DELLEY B. Dmol3 DFT studies: from molecules and molecular environments to surfaces and solids[J]. Comp Mater Sci,2000,17(2):122−126. [23] PERDEW J P, BURKE K, WANG Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system[J]. Phys Rev B Condens Matter,1996,54(23):16533−16539. doi: 10.1103/PhysRevB.54.16533 [24] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys. Rev. Lett,1996,77(18):3865−3868. doi: 10.1103/PhysRevLett.77.3865 [25] NOLAN M, GRIGOLEIT S, SAYLE D C, et al. Density functional theory studies of the structure and electronic structure of pure and defective low index surfaces of ceria[J]. Surf. Sci,2005,576(1-3):217−229. doi: 10.1016/j.susc.2004.12.016 [26] YANG Y, HU K, ZHANG J, et al. Adsorption properties of noble-metal (Ag, Rh, or Au)-doped CeO2(110) to CO: a DFT + U study[J]. Comput. Mater. Sci,2024,231:112543. doi: 10.1016/j.commatsci.2023.112543 [27] HE P, WU J, JIANG X, et al. Effect of SO3 on elemental mercury adsorption on a carbonaceous surface[J]. Appl. Surf. Sci,2012,258(22):8853−8860. doi: 10.1016/j.apsusc.2012.05.104 [28] YANG Y, LIU J, ZHANG B, et al. Density functional theory study on the heterogeneous reaction between Hg0 and Hcl over spinel-type MnFe2O4[J]. Chem. Eng. J,2017,308:897−903. doi: 10.1016/j.cej.2016.09.128 [29] 李宗宝, 贾礼超, 王霞等. N、C掺杂比例对锐钛矿TiO2电子结构影响的第一性原理研究[J]. 黑龙江大学工程学报,2014,5(1):41−46.LI Zongbao, JIA Lichao, WANG Xia, et al. Density function theory on the electronic structure property of anatase TiO2 doped by N or C with different percents[J]. J. Eng. HeiLongJiang. Univ,2014,5(1):41−46. [30] LYU Z, NIU S, LU C, et al. A density functional theory study on the selective catalytic reduction of NO by NH3 reactivity of α-Fe2O3 (001) catalyst doped by Mn, Ti, Cr and Ni[J]. Fuel,2020,267:117147. doi: 10.1016/j.fuel.2020.117147 [31] HUANG X, ZHANG K, PENG B, et al. Ceria-based materials for thermocatalytic and photocatalytic organic synthesis[J]. ACS Catal,2021,11(15):9618−9678. doi: 10.1021/acscatal.1c02443 [32] YANG Z X, YU X H, LU Z S, et al. Oxygen vacancy pairs on CeO2(110): A DFT + U study[J]. Phys. Lett. A,2009(373):312786−2792. [33] REN D, GUI K. Study of the adsorption of NH3 and NO x on the nanoγ-Fe2O3 (001) surface with density functional theory[J]. Appl. Surf. Sci,2019,487:171−179. doi: 10.1016/j.apsusc.2019.04.250 [34] LI L, SONG L, ZHANG X, et al. Effect of substitutional and interstitial boron-doped NiCO2S4 on the electronic structure and surface adsorption: high rate and long-term stability[J]. ACS Appl. Energy Mater,2022,5(2):2505−2513. doi: 10.1021/acsaem.1c04033 [35] HU P, WENG Q, LI D, et al. Effects of O2, SO2, H2O and CO2 on As2O3 adsorption by γ-Al2O3 based on DFT analysis[J]. J. Hazard. Mater,2021,403:123866. doi: 10.1016/j.jhazmat.2020.123866 [36] XU H X, CHENG D J, CAO D P, et al. A universal principle for a rational design of single-atom electrocatalysts[J]. Nat. Catal,2018,1:339−348. doi: 10.1038/s41929-018-0063-z [37] WU Y, ZHOU X, MI T, et al. Theoretical insight into the interaction mechanism between V2O5/TiO2 (001) surface and arsenic oxides in flue gas[J]. Appl. Surf. Sci,2021,535:147752. doi: 10.1016/j.apsusc.2020.147752 [38] 张佳松, 王辉, 王宁等. CO在不同氧缺陷Cu1/CeO2(110)表面的吸附: DFT+U[J]. 燃料化学学报,2022,50(3):326−336. doi: 10.1016/S1872-5813(21)60149-4ZHANG Jiasong, WANG Hui, WANG ning, et al. Adsorption of CO on Cu1/CeO2(110) surface with different oxygen defects: DFT + U[J]. J. Fuel Chem. Technol,2022,50(3):326−336. doi: 10.1016/S1872-5813(21)60149-4 [39] LIU Z, YU F, DONG D, et al. Transition-metal‐doped ceria carried on two-dimensional vermiculite for selective catalytic reduction of NO with CO: experiments and density functional theory[J]. Appl. Surf. Sci,2021,566:150704. doi: 10.1016/j.apsusc.2021.150704 -




 下载:
下载: