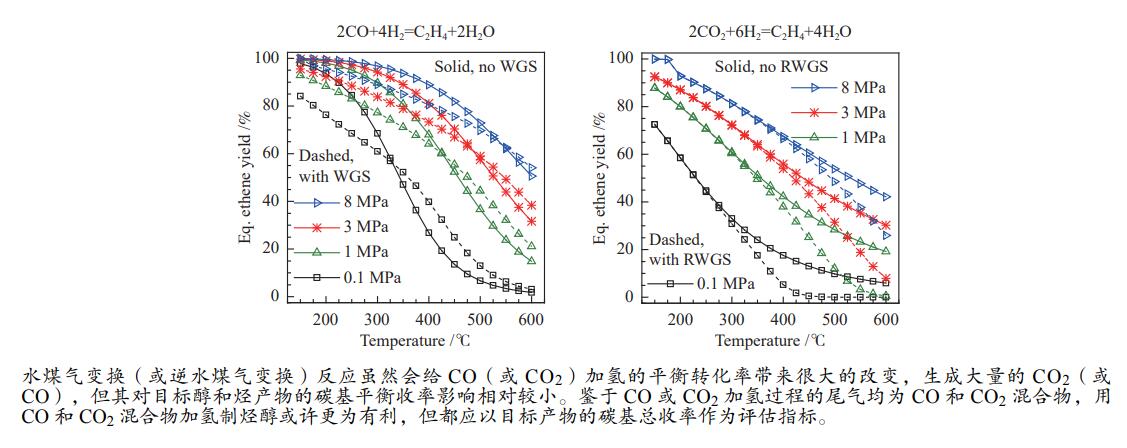
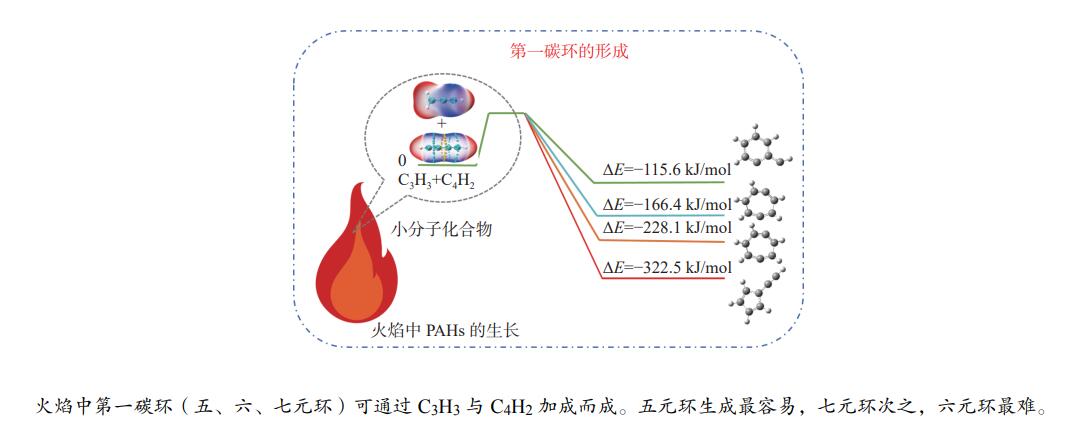
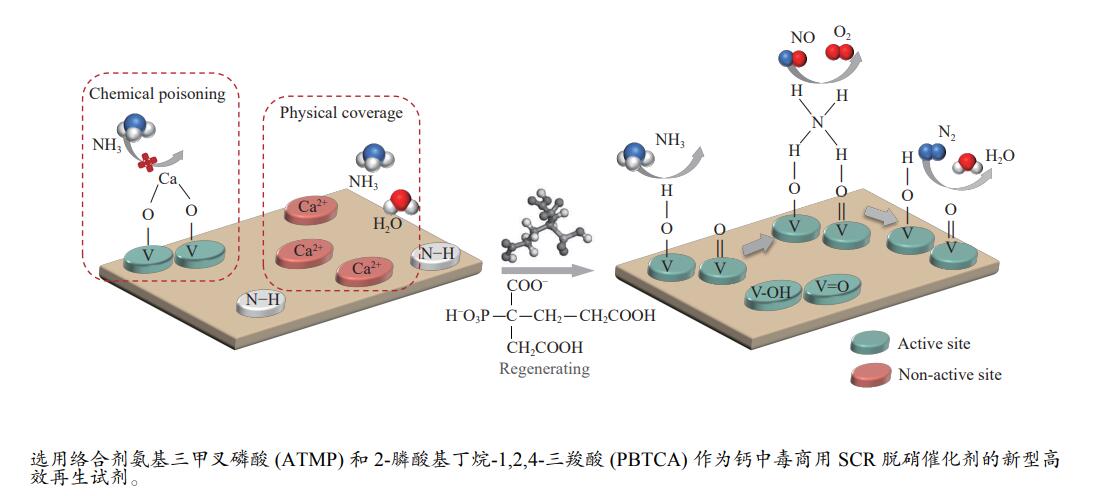
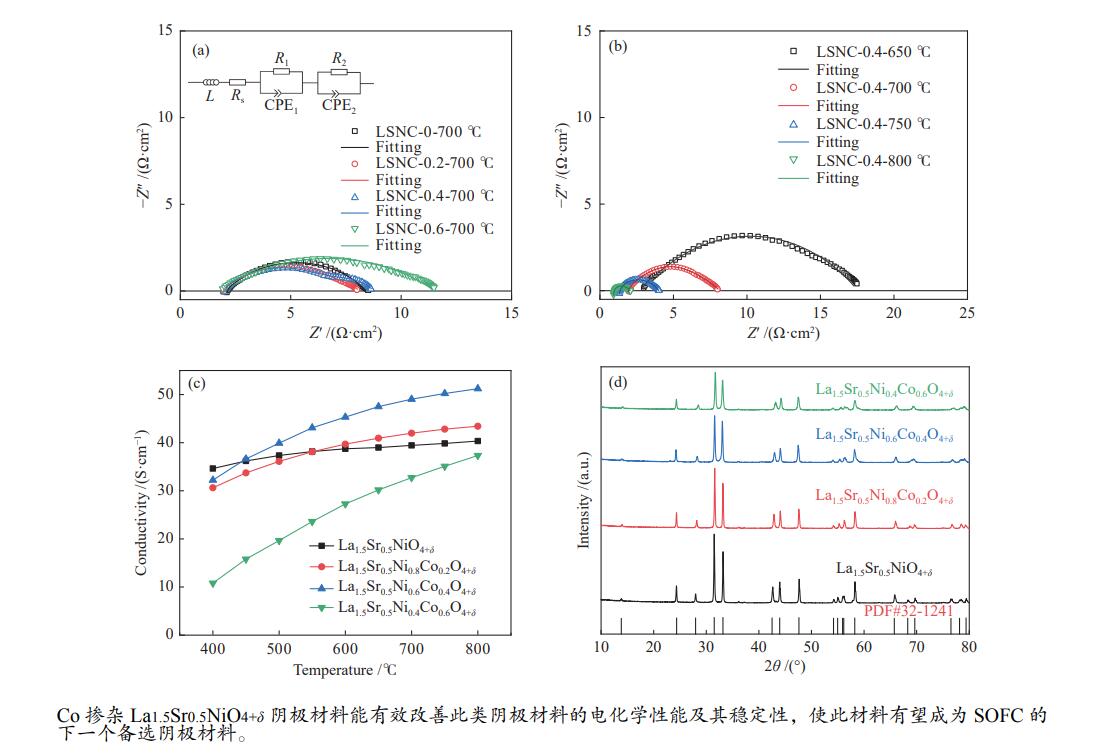
2023 Vol. 51, No. 4
Display Method:
2023, 51(4): 415-427.
doi: 10.1016/S1872-5813(22)60047-1
Abstract:
2023, 51(4): 428-443.
doi: 10.19906/j.cnki.JFCT.2022063
Abstract:
2023, 51(4): 444-457.
doi: 10.19906/j.cnki.JFCT.2022061
Abstract:
2023, 51(4): 458-472.
doi: 10.19906/j.cnki.JFCT.2022067
Abstract:
2023, 51(4): 473-481.
doi: 10.1016/S1872-5813(22)60050-1
Abstract:
Abstract:
2023, 51(4): 492-501.
doi: 10.1016/S1872-5813(22)60054-9
Abstract:
2023, 51(4): 502-510.
doi: 10.19906/j.cnki.JFCT.2022068
Abstract:
2023, 51(4): 511-518.
doi: 10.1016/S1872-5813(22)60057-4
Abstract:
2023, 51(4): 519-527.
doi: 10.19906/j.cnki.JFCT.2022094
Abstract:
2023, 51(4): 528-537.
doi: 10.19906/j.cnki.JFCT.2022069
Abstract:
2023, 51(4): 538-543.
doi: 10.19906/j.cnki.JFCT.2022054
Abstract:
2023, 51(4): 544-553.
doi: 10.19906/j.cnki.JFCT.2022070
Abstract:
2023, 51(4): 554-561.
doi: 10.1016/S1872-5813(22)60059-8
Abstract:
2023, 51(4): 562-570.
doi: 10.19906/j.cnki.JFCT.2022060
Abstract: