Selective xylose hydrogenolysis to 1,2-diols using Co@NC catalysts
-
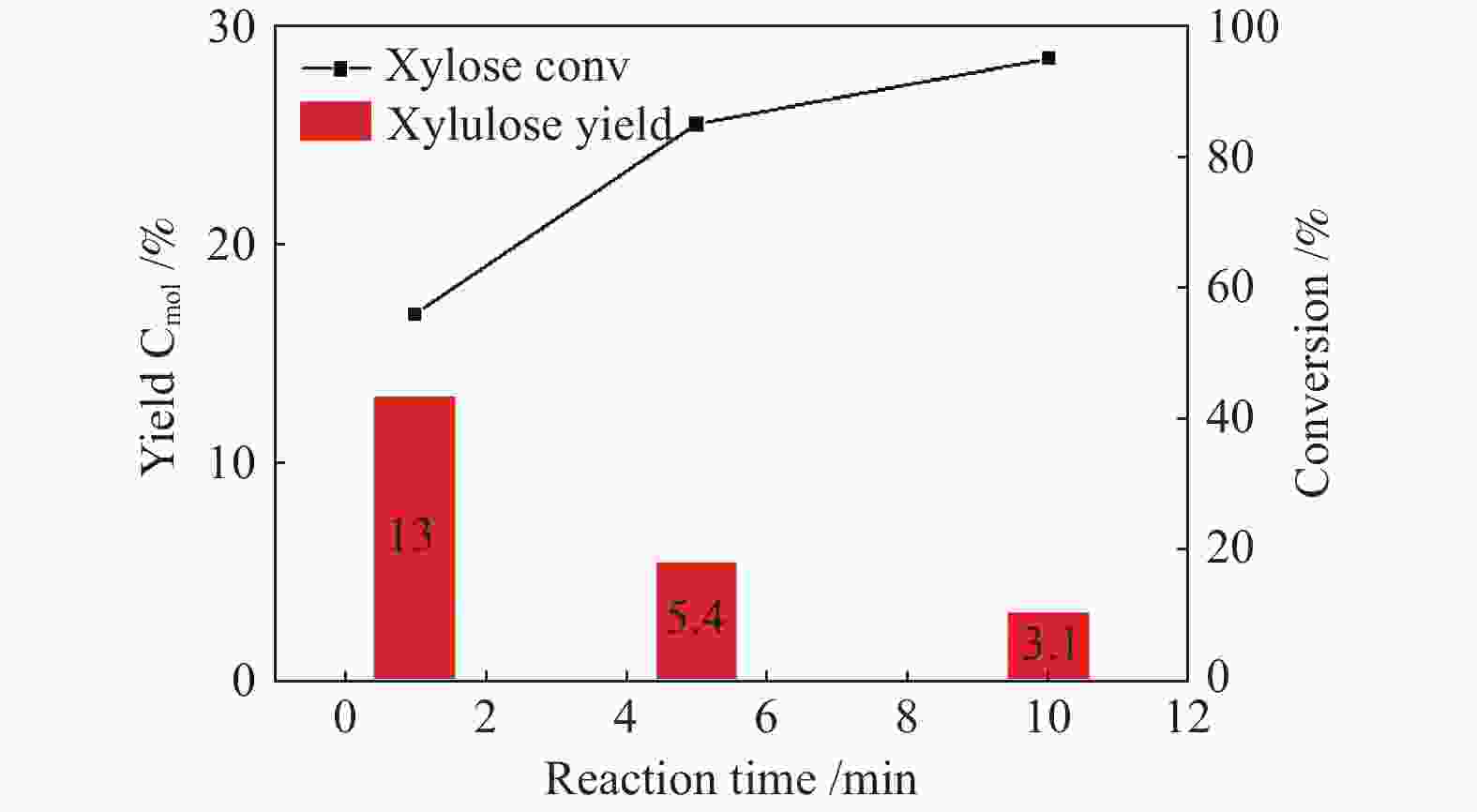
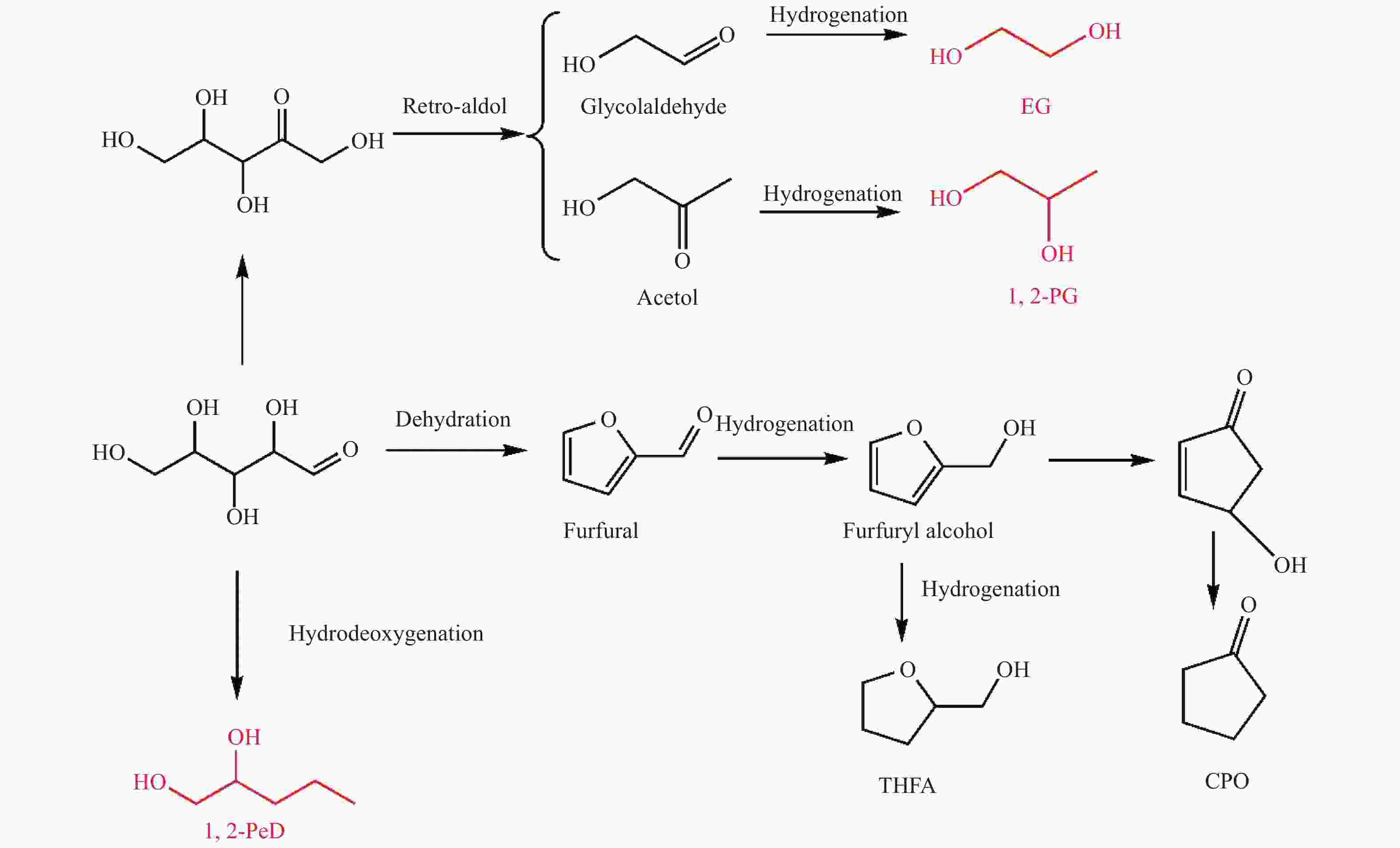
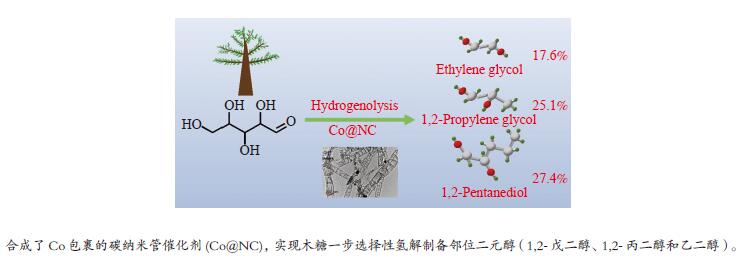
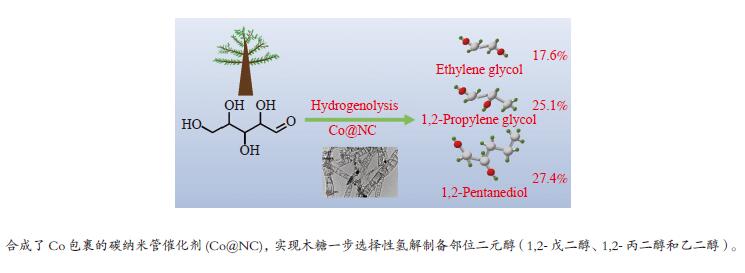
摘要: 本研究采用bottom-up法,制备了具有加氢和异构活性的碳包裹金属催化剂Co@NC,用于催化木糖氢解制备1,2-二元醇。结合XRD、TEM、XPS等表征手段对比了不同焙烧温度制备的Co@NC催化剂的物理和化学性质。研究发现,600 ℃焙烧的Co@NC催化剂具有最高的二元醇的总收率 (70.1%),其中,乙二醇、1,2-丙二醇和1,2-戊二醇的收率分别达到17.6%、25.1%和27.4%。机理研究表明,N的掺杂为Co@NC提供了碱性位点,在碱的催化作用下促进木糖向木酮糖的异构,再通过Retro-aldol反应得到乙醇醛和丙酮醇中间产物,最后经加氢得到乙二醇和1,2-丙二醇。1,2-戊二醇来源于木糖的加氢脱氧,其产率高于文献报道的最佳结果。本研究工作发展的水热稳定性优异的Co@NC催化剂为生物质高效制备1,2-二元醇提供了新的研究思路。Abstract: Xylose is the predominant component of hemicellulose, and converting xylose to valuable compounds is essential to achieve biomass utilization. Herein, N-doped carbon nanotubes encapsulated metal catalysts (Co@NC) with hydrogenation and isomerization capacities were synthesized via bottom-up method for catalyzing xylose hydrogenolysis into 1,2-diols. The physicochemical properties of Co@NC prepared with different calcination temperature were determined by XRD, TEM, XPS and so on. The Co@NC prepared at 600 ℃ exhibited the optimal catalytic activity, and the yield of diols reached 70.1% with ethylene glycol, 1,2-propylene glycol and 1,2-pentanediol being 17.6%, 25.1% and 27.4%, respectively. The doping N species served as the basic sites which benefited the isomerization of xylose to xylulose. Xylulose was subsequently converted to glycolaldehyde and acetol through Retro-aldol reaction, followed by hydrogenation to produce ethylene glycol and 1,2-propylene glycol. 1,2-Pentanediol derived from the selective hydrodeoxygenation of xylose, the yield of which surpassed the results that had been reported. The Co@NC catalysts with high robustness under harsh hydrothermal conditions provided new insights into the effective conversion of lignocellulosic biomass to 1,2-diols.
-
Key words:
- Co@NC /
- xylose /
- hydrogenolysis /
- 1,2-diols /
- 1,2-pentanediol
-
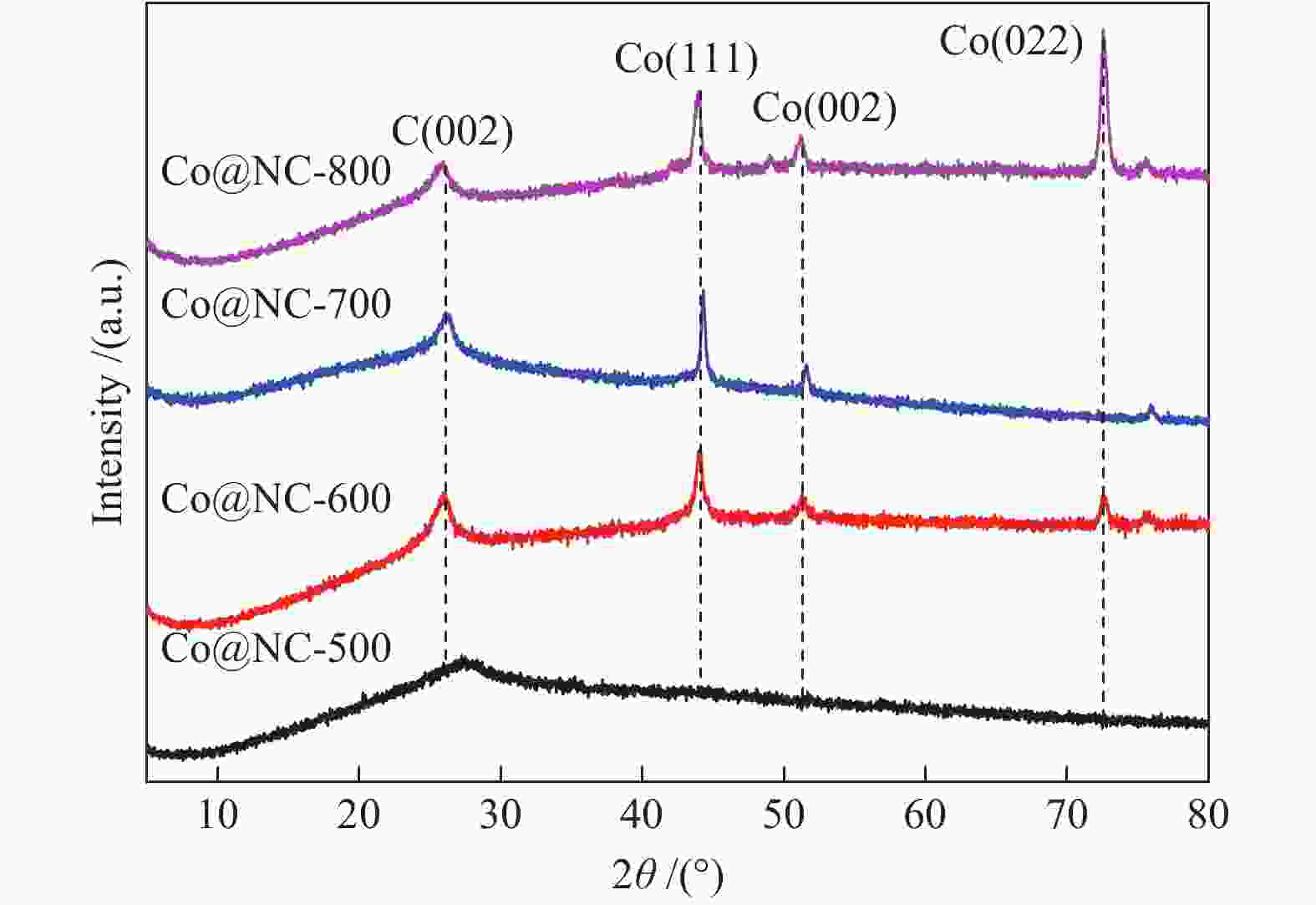
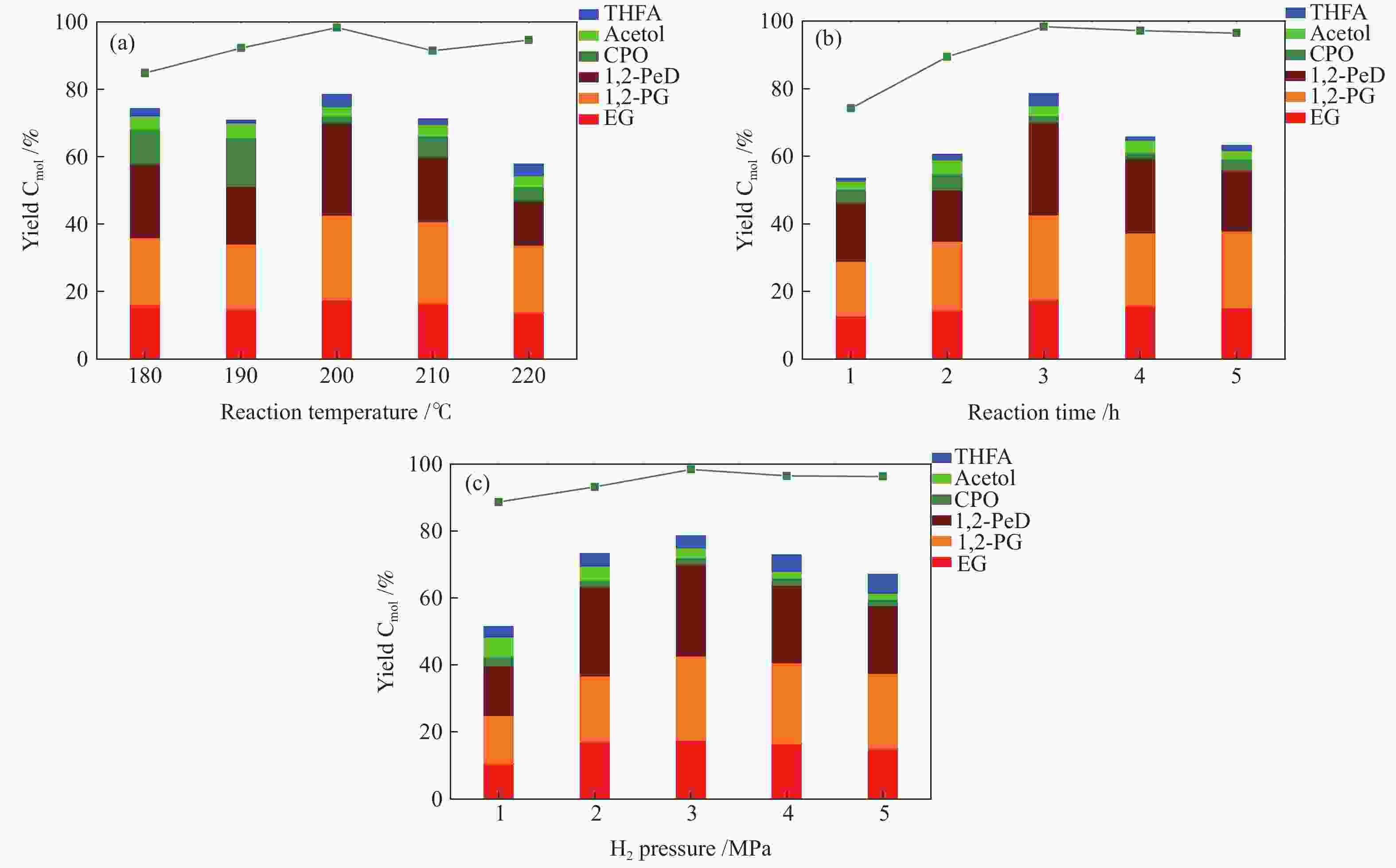
图 10 Co@NC-600催化剂上不同反应条件下木糖氢解反应性能
Figure 10 Effect of (a) reaction temperature, (b) reaction time and (c) H2 pressure on the selective hydrogenolysis of xylose over Co@NC-600
Reaction conditions: 0.2 g xylose, 0.07 g catalysts, 20 mL de-ionized water, EG: ethylene glycol; 1,2-PG: 1,2-propylene glycol; 1,2-PeD: 1,2-pentanediol; CPO: cyclopentanone; THFA: tetrahydrofurfuryl alcohol
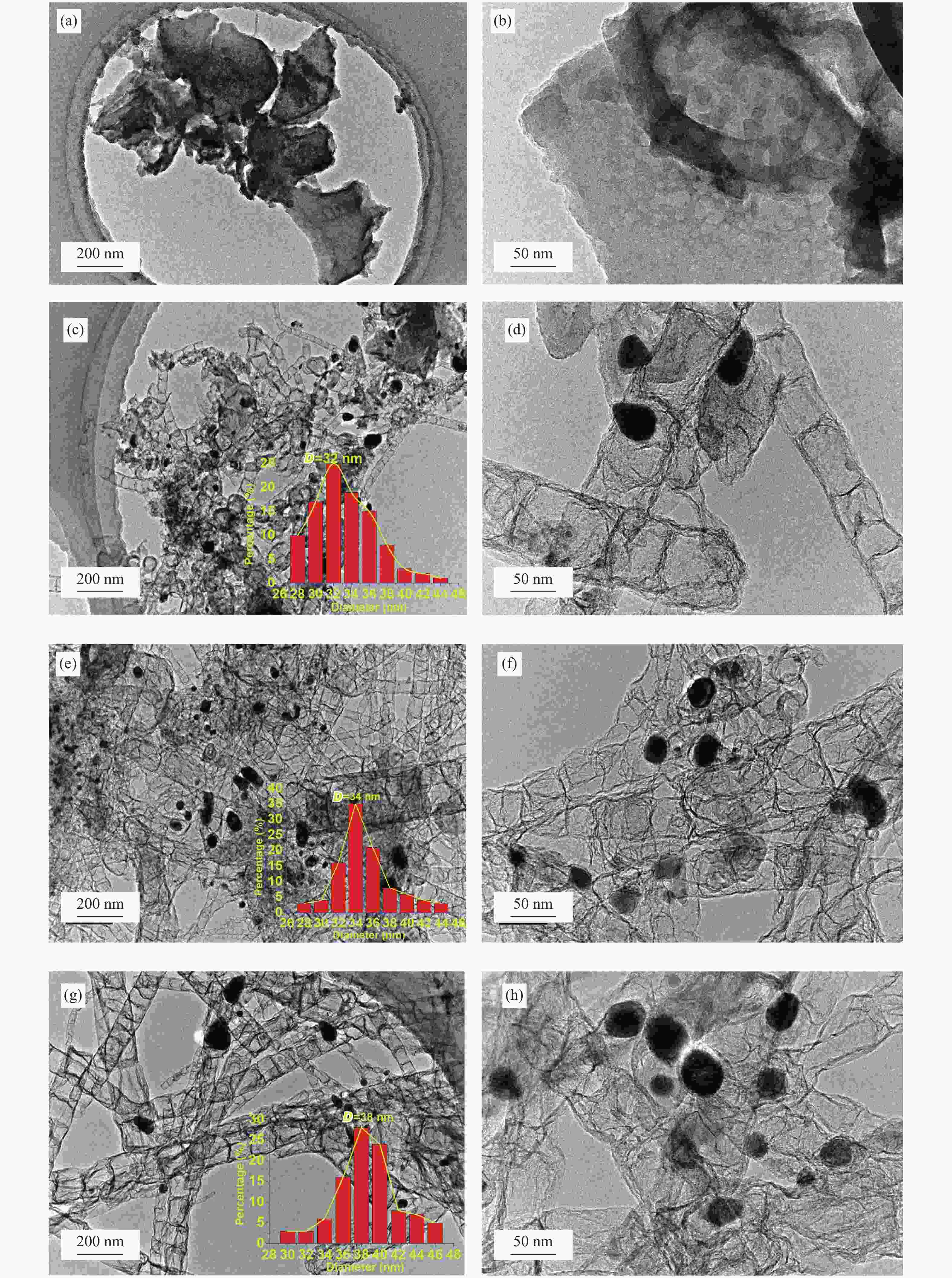
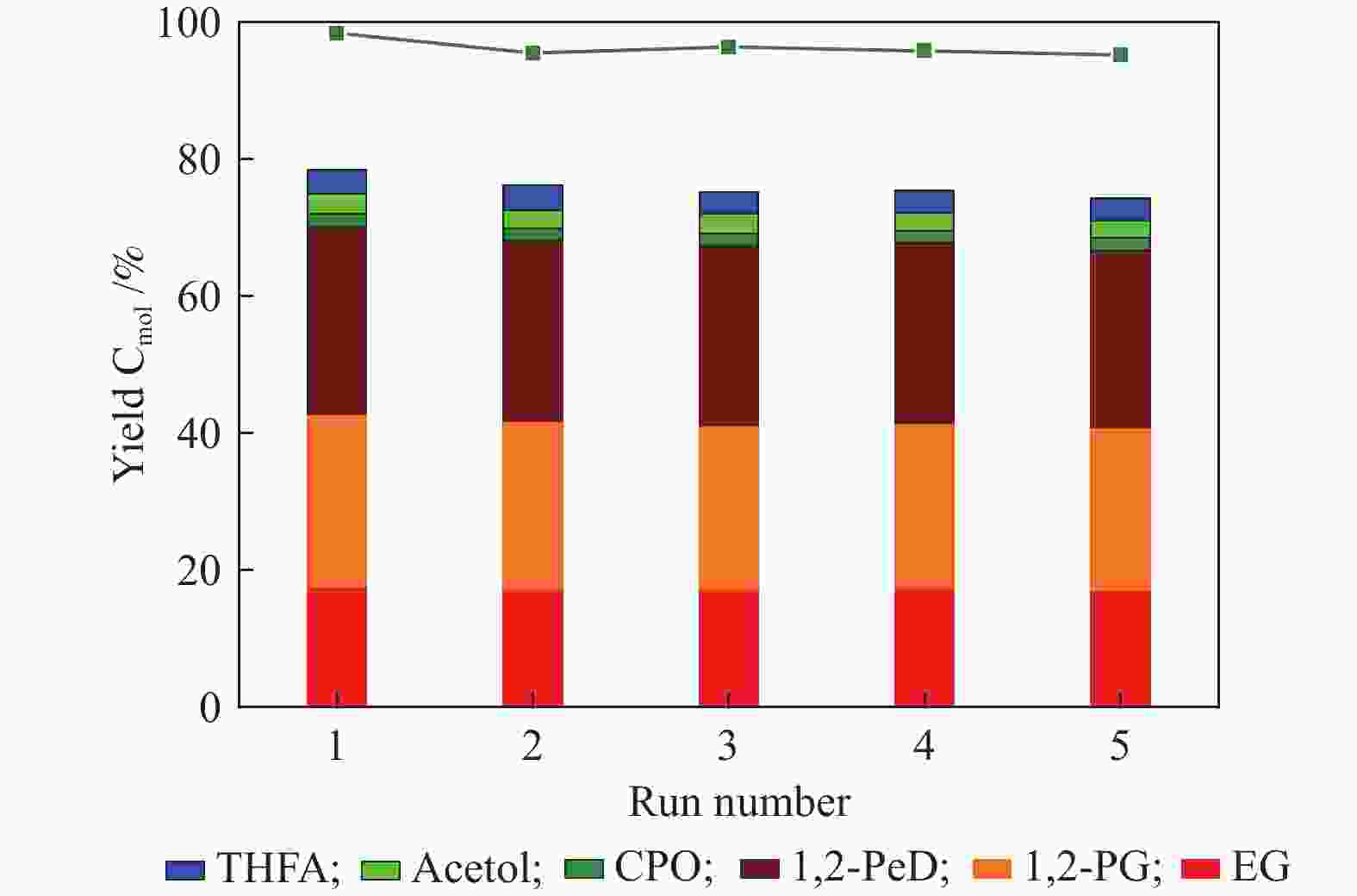
图 11 Co@NC催化木糖转化的循环使用性能
Figure 11 Cycle test of Co@NC for xylose conversion.
Reaction conditions: 0.2 g xylose, 0.07 g catalysts, 20 mL de-ionized water, reaction temperature: 200 ℃, reaction time: 3 h, H2 pressure: 3 MPa. EG: ethylene glycol; 1,2-PG: 1,2-propylene glycol; 1,2-PeD: 1,2-pentanediol; CPO: cyclopentanone; Ac: acetol; THFA: tetrahydrofurfuryl alcohol
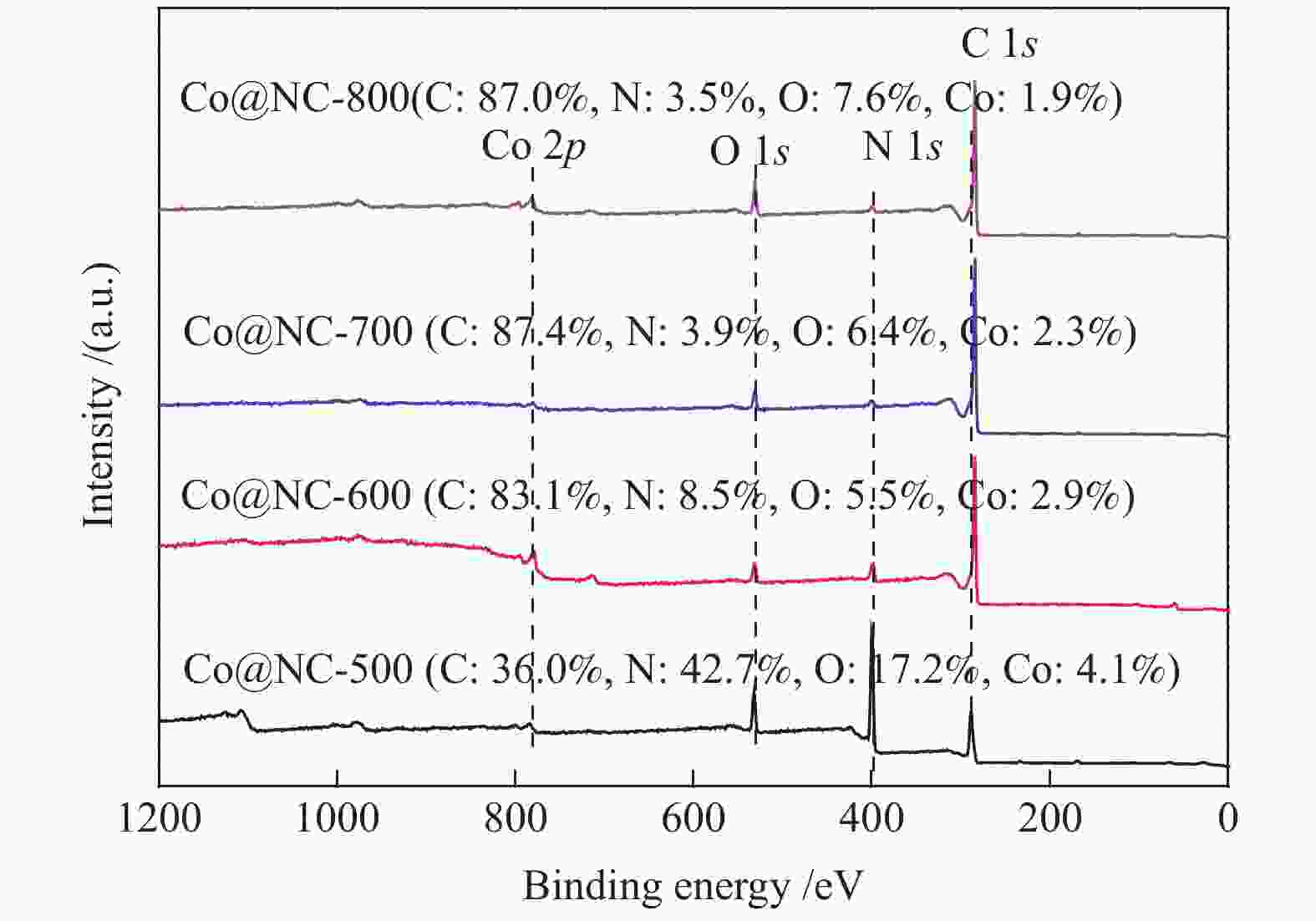
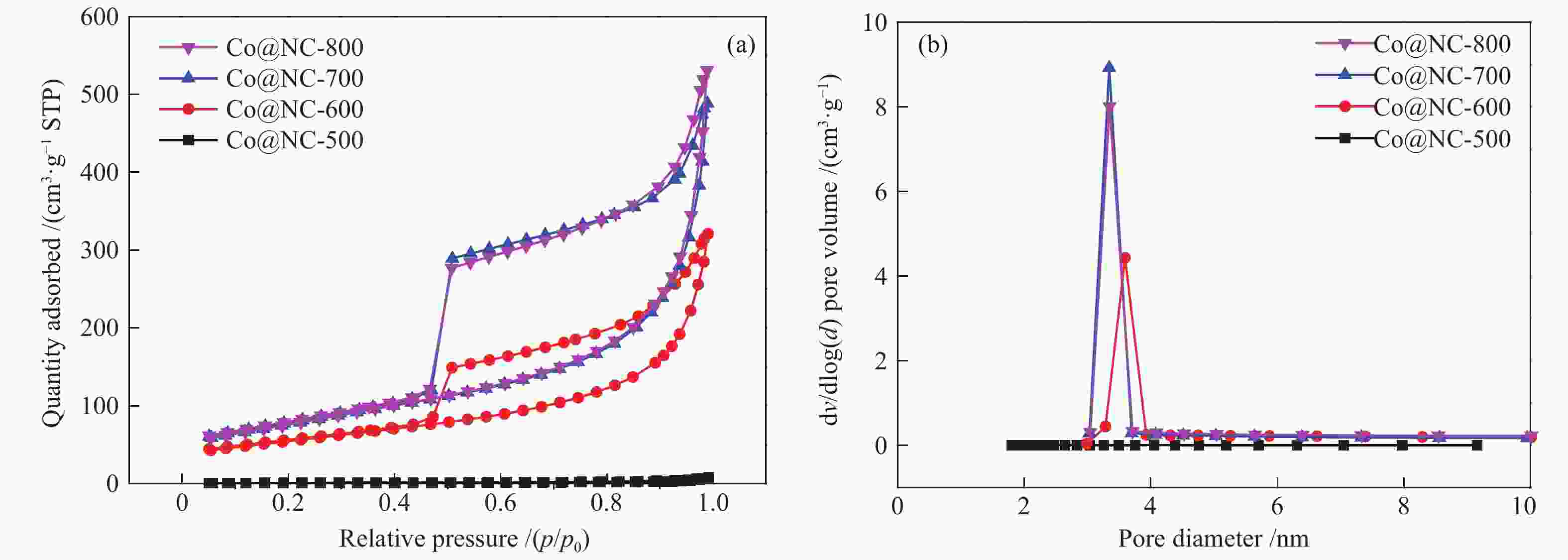
表 1 Co@NC催化剂的BET数据和孔结构参数
Table 1 BET surface area and pores of Co@NC catalysts
Catalyst BET surface area/(m2·g−1) Pore volume/(cm3·g−1) Pore size/nm Co@NC-500 3.4 0.01 – Co@NC-600 194.0 0.50 3.6 Co@NC-700 275.2 0.76 3.3 Co@NC-800 275.3 0.83 3.4 表 2 Co@NC催化剂的表面酸/碱量
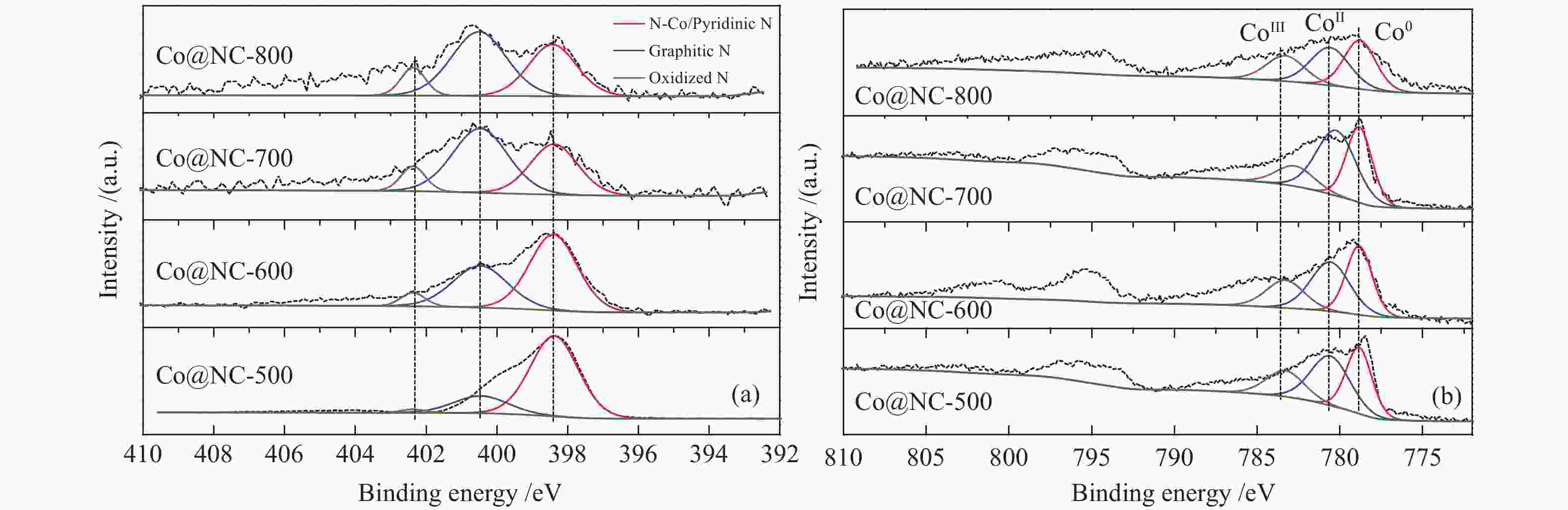
Table 2 Surface acid/base amounts of Co@NC catalysts
Catalyst Total acid amount/
(mmol·g−1)Weak base amount/
(mmol·g−1)Strong base amount/
(mmol·g−1)Total base amount/
(mmol·g−1)Co@NC-500 2.132 0.118 8.694 8.812 Co@NC-600 1.142 0.221 3.681 3.902 Co@NC-700 1.104 0.163 1.402 1.565 Co@NC-800 0.586 0.155 0.997 1.152 Ni@NC-600 3.162 0.218 4.649 4.867 表 3 不同N掺杂碳包裹型催化剂在木糖氢解反应中的催化性能
Table 3 Xylose conversion to 1,2-diols over different N-doped carbon encapsulated metal catalysts
Catalyst Conv./% Yield Cmol/% EG 1,2-PG 1,2-PeD CPO Ac THFA Co@NC-500 37.3 0 0 0 0 0 0 Co@NC-600 98.4 17.6 25.1 27.4 2.0 2.9 3.5 Co@NC-700 95.1 12.9 11.8 23.4 9.3 17.4 1.8 Co@NC-800 90.9 6.0 3.5 4.3 3.7 26.1 1.0 Ni@NC-600 82.4 2.4 8.2 0 15.0 20.5 10.3 Fe@NC-600 62.9 0 0 0 0 10.5 0 Reaction conditions: 0.2 g xylose, 0.07 g catalysts, 20 mL de-ionized water, reaction temperature: 200 ℃, reaction time: 3 h, H2 pressure: 3 MPa. EG: ethylene glycol; 1,2-PG: 1,2-propylene glycol; 1,2-PeD: 1,2-pentanediol; CPO: cyclopentanone; Ac: acetol; THFA: tetrahydrofurfuryl alcohol 表 4 不同催化剂上木糖的氢解性能
Table 4 Xylose hydrogenolysis catalyzed by different catalysts
Catalyst Conv./% Yield Cmol/% xylitol cyclopentanone acetol Co@C 97.5 96.5 0 0 Ni@C 93.7 90.7 0 0 Fe@C 96.4 85.0 0 0 Co/C 99.9 99.9 0 0 N-AC 62.1 0 13.8 20.4 Reaction conditions: 0.2 g xylose, 0.07 g catalysts, 20 mL de-ionized water, reaction temperature: 200 ℃, reaction time: 3 h, H2 pressure: 3 MPa, N-AC: N-activated carbon 表 5 1,2-PeD收率与文献对比
Table 5 Comparison of 1,2-PeD yield in this work with reported references
表 6 不同原料下的产物分布
Table 6 Product distribution using various possible intermediates
Substrate Conv./% Yield Cmol/% EG 1,2-PG 1,2-PeD CPO cyclopentanol Ac THFA Xylitol 25.2 1.9 0.8 2.5 0 0 0 0 Xylulose 92.5 24.4 32.9 7.5 1.0 1.1 8.5 0.5 Glycolaldehyde 99.9 93.7 0 0 0 0 0 0 Acetol 98.2 0 95.3 0 0 0 0 0 Methylglyoxal 95.6 0 39.1 0 0 0 19.3 0 Furfural 99.9 0 0 0 86.5 10.1 0 0.3 Furfuryl alcohol 99.9 0 0 0 78.2 13.8 0 3.6 Glucose 93.5 0 0 0 0 0 8.9 0 Fructose 96.7 0 10.4 0 0 0 30.1 0 Reaction conditions: 0.2 g xylose, 0.07 g catalysts, 20 mL de-ionized water, reaction temperature: 200 ℃, reaction time: 3 h, H2 pressure: 3 MPa. EG: ethylene glycol; 1,2-PG: 1,2-propylene glycol; 1,2-PeD: 1,2-pentanediol; CPO: cyclopentanone; Ac: acetol; THFA: tetrahydrofurfuryl alcohol -
[1] KAUR M, KUMAR M, SACHDEVA S, PURI S K. Aquatic weeds as the next generation feedstock for sustainable bioenergy production[J]. Bioresour Technol,2018,251:390−402. doi: 10.1016/j.biortech.2017.11.082 [2] ZHAO W, ZHAO F, ZHANG S, GONG Q, CHEN G. Ethanol production by simultaneous saccharification and cofermentation of pretreated corn stalk[J]. J Basic Microbiol,2019,59(7):744−753. doi: 10.1002/jobm.201900117 [3] 许彦娟, 糠醛催化加氢制备1, 2-戊二醇的研究[D]. 杭州: 浙江大学, 2014.XU Yan-juan, Study on catalytic hydrogenolysis of furfuryl alcohol into 1, 2-pentanediol[D]. Hangzhou: Zhejiang University, 2014. [4] ORDOMSKY V V, SCHOUTEN J C, VAN DER SCHAAF J, NIJHUIS T A. Biphasic single-reactor process for dehydration of xylose and hydrogenation of produced furfural[J]. Appl Catal A: Gen,2013,451:6−13. doi: 10.1016/j.apcata.2012.11.013 [5] WANG N, CHEN Z, LIU L. Acid catalysis dominated suppression of xylose hydrogenation with increasing yield of 1,2-pentanediol in the acid-metal dual catalyst system[J]. Appl Catal A: Gen,2018,561:41−48. doi: 10.1016/j.apcata.2018.05.019 [6] SUN J, LIU H. Selective hydrogenolysis of biomass-derived xylitol to ethylene glycol and propylene glycol on supported Ru catalysts[J]. Green Chem,2011,13(1):135−142. doi: 10.1039/C0GC00571A [7] VIJAYA SHANTHI R, MAHALAKSHMY R, THIRUNAVUKKARASU K, SIVASANKER S. Hydrogenolysis of sorbitol over Ni supported on Ca- and Ca(Sr)-hydroxyapatites[J]. Mol Catal,2018,451:170−177. doi: 10.1016/j.mcat.2017.12.031 [8] DENG J, DENG D, BAO X. Robust catalysis on 2D materials encapsulating metals: Concept, application, and perspective[J]. Adv Mater,2017,29(43):2100−2104. [9] GONG W, CHEN C, ZHANG H, WANG G, ZHAO H. Highly dispersed Co and Ni nanoparticles encapsulated in N-doped carbon nanotubes as efficient catalysts for the reduction of unsaturated oxygen compounds in aqueous phase[J]. Catal Sci Technol,2018,8(21):5506−5514. doi: 10.1039/C8CY01488D [10] AIJAZ A MASA J, ROSLER C, XIA W, WEIDE P, BOTZ A J, FISCHER R A, SCHUHMANN W, MUHLER M. Co@Co3O4 encapsulated in carbon nanotube-grafted nitrogen-doped carbon polyhedra as an advanced bifunctional oxygen electrode[J]. Angew Chem Int Ed Eng,2016,55(12):4087−4091. doi: 10.1002/anie.201509382 [11] XU X. A general metal-organic framework (MOF)-derived selenidation strategy for in situ carbon-encapsulated metal selenides as high-rate anodes for Na-ion batteries[J]. Adv Funct Mater,2018,28(16):1707573. doi: 10.1002/adfm.201707573 [12] TU Y, REN P, DENG D, BAO X. Structural and electronic optimization of graphene encapsulating binary metal for highly efficient water oxidation[J]. Nano Energy,2018,52:494−500. doi: 10.1016/j.nanoen.2018.07.062 [13] FANG L J, WANG X L, LI Y H, LIU P F, WANG Y L, ZENG H D, YANG H G. Nickel nanoparticles coated with graphene layers as efficient co-catalyst for photocatalytic hydrogen evolution[J]. Appl Catal B: Environ,2017,200:578−584. doi: 10.1016/j.apcatb.2016.07.033 [14] AI L, SU J, WANG M, JIANG J. Bamboo-structured nitrogen-doped carbon nanotube coencapsulating cobalt and molybdenum carbide nanoparticles: An efficient bifunctional electrocatalyst for overall water splitting[J]. ACS Sustainable Chem Eng,2018,6(8):9912−9920. doi: 10.1021/acssuschemeng.8b01120 [15] DUAN X, AO Z, SUN H, INDRAWIRAWAN S, WANG Y, KANG J, LIANG F, ZHU Z H, WANG S. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis[J]. ACS Appl Mater Inter,2015,7(7):4169−4178. doi: 10.1021/am508416n [16] LIU B, DAI W, LIANG Z, YE J, OUYANG L. Fe/N/C carbon nanotubes with high nitrogen content as effective non-precious catalyst for oxygen reduction reaction in alkaline medium[J]. Int J Hydrogen Energy,2017,42(9):5908−5915. doi: 10.1016/j.ijhydene.2016.12.043 [17] FENG X, BO X, GUO L. CoM(M=Fe,Cu,Ni)-embedded nitrogen-enriched porous carbon framework for efficient oxygen and hydrogen evolution reactions[J]. J Power Sources,2018,389:249−259. doi: 10.1016/j.jpowsour.2018.04.027 [18] KANG J, DUAN X, WANG C, SUN H, TAN X, TADE M O, WANG S. Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability[J]. Chem Eng J,2018,332:398−408. doi: 10.1016/j.cej.2017.09.102 [19] ZHUANG M, OU X, DOU Y, ZHANG L, ZHANG Q, WU R, DING Y, SHAO M, LUO Z. Polymer-embedded Fabrication of Co 2P nanoparticles encapsulated in N,P-doped graphene for hydrogen generation[J]. Nano Lett,2016,16(7):4691−4698. doi: 10.1021/acs.nanolett.6b02203 [20] SI Y, ZHANG Y, LU L, ZHANG S, CHEN Y, LIU J, JIN H, HOU S, DAI K, SONG W. Boosting visible light photocatalytic hydrogen evolution of graphitic carbon nitride via enhancing it interfacial redox activity with cobalt/nitrogen doped tubular graphitic carbon[J]. Appl Catal B: Environ,2018,225:512−518. doi: 10.1016/j.apcatb.2017.12.010 [21] YAO Y, CHEN H, LIAN C, WEI F, ZHANG D, WU G, CHEN B, WANG S. Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal[J]. J Hazard Mater,2016,314:129−139. doi: 10.1016/j.jhazmat.2016.03.089 [22] LIU Q, WANG H, XIN H, WANG C, YAN L, WANG Y ZHANG Q, ZHANG X, XU Y, HUBER G W, MA L. Selective cellulose hydrogenolysis to ethanol using Ni@C combined with phosphoric acid catalysts[J]. ChemSusChem,2019,12(17):3977−3987. doi: 10.1002/cssc.201901110 [23] ZENG M, LIU Y, ZHAO F, NIE K, HAN N, WANG X, HUANG W, SONG X, ZHONG J, LI Y. Metallic cobalt nanoparticles encapsulated in nitrogen-enriched graphene shells: Its bifunctional electrocatalysis and application in zinc-air batteries[J]. Adv Funct Mater,2016,26(24):4397−4404. doi: 10.1002/adfm.201600636 [24] ZHANG X, LIU S, ZANG Y, LIU R, LIU G, WANG G, ZHANG Y, ZHANG H, ZHAO H. Co/Co9S8@S, N-doped porous graphene sheets derived from S, N dual organic ligands assembled Co-MOFs as superior electrocatalysts for full water splitting in alkaline media[J]. Nano Energy,2016,30:93−102. doi: 10.1016/j.nanoen.2016.09.040 [25] MO Z, XU H, CHEN Z, SHE X, SONG Y, LIAN J, ZHU X, YAN P, LEI Y, YUAN S, LI H. Construction of MnO2/Monolayer g-C3N4 with Mn vacancies for Z-scheme overall water splitting[J]. Appl Catal B: Environ,2019,241:452−460. doi: 10.1016/j.apcatb.2018.08.073 [26] XIU Z, WANG H, CAI C, LI C, YAN L, WANG C, LI W, XIN H, ZHU C, ZHANG Q, LIU Q, MA L. Ultrafast glycerol conversion to lactic acid over magnetically recoverable Ni-NiOx@C catalysts[J]. Ind Eng Chem Res,2020,59(21):9912−9925. doi: 10.1021/acs.iecr.0c01145 [27] JIN L, ZHAO X, QIAN X, DONG M. Nickel nanoparticles encapsulated in porous carbon and carbon nanotube hybrids from bimetallic metal-organic-frameworks for highly efficient adsorption of dyes[J]. J Colloid Interface Sci,2018,509:245−253. doi: 10.1016/j.jcis.2017.09.002 [28] LIU C, SHANG Y, QI H, WANG X, GUI J, ZHANG C, ZHU Y, LI Y. Effect of the ZrO2 phase on Pd-based bifunctional catalysts for the hydrogenolysis of glucose[J]. Catal Commun,2019,128:105688. doi: 10.1016/j.catcom.2019.04.020 [29] FANG R, LIU H, LUQUE R, LI Y. Efficient and selective hydrogenation of biomass-derived furfural to cyclopentanone using Ru catalysts[J]. Green Chem,2015,17(8):4183−4188. doi: 10.1039/C5GC01462J [30] LANGE J P, WADMAN S H. Furfural to 1,4-butanediol/tetrahydrofuran-a detailed catalyst and process design[J]. ChemSusChem,2020,13(19):5329−5337. doi: 10.1002/cssc.202001376 [31] CORMA A, DE LA TORRE O, RENZ M, VILLANDIER N. Production of high-quality diesel from biomass waste products[J]. Angew Chem Int Ed Eng,2011,50(10):2375−2378. doi: 10.1002/anie.201007508 [32] SONG H, WANG P, LI S, DENG W, LI Y, ZHANG Q, WANG Y. Direct conversion of cellulose into ethanol catalysed by a combination of tungstic acid and zirconia-supported Pt nanoparticles[J]. Chem Commun (Camb),2019,55(30):4303−4306. doi: 10.1039/C9CC00619B [33] GU M, SHEN Z, YANG L, DONG W, KONG L, ZHANG W, PENG B Y, ZHANG Y. Reaction route selection for cellulose hydrogenolysis into C2/C3 glycols by ZnO-modified Ni-W/beta-zeolite catalysts[J]. Sci Rep,2019,9(1):11938. doi: 10.1038/s41598-019-48103-6 -




 下载:
下载: