-
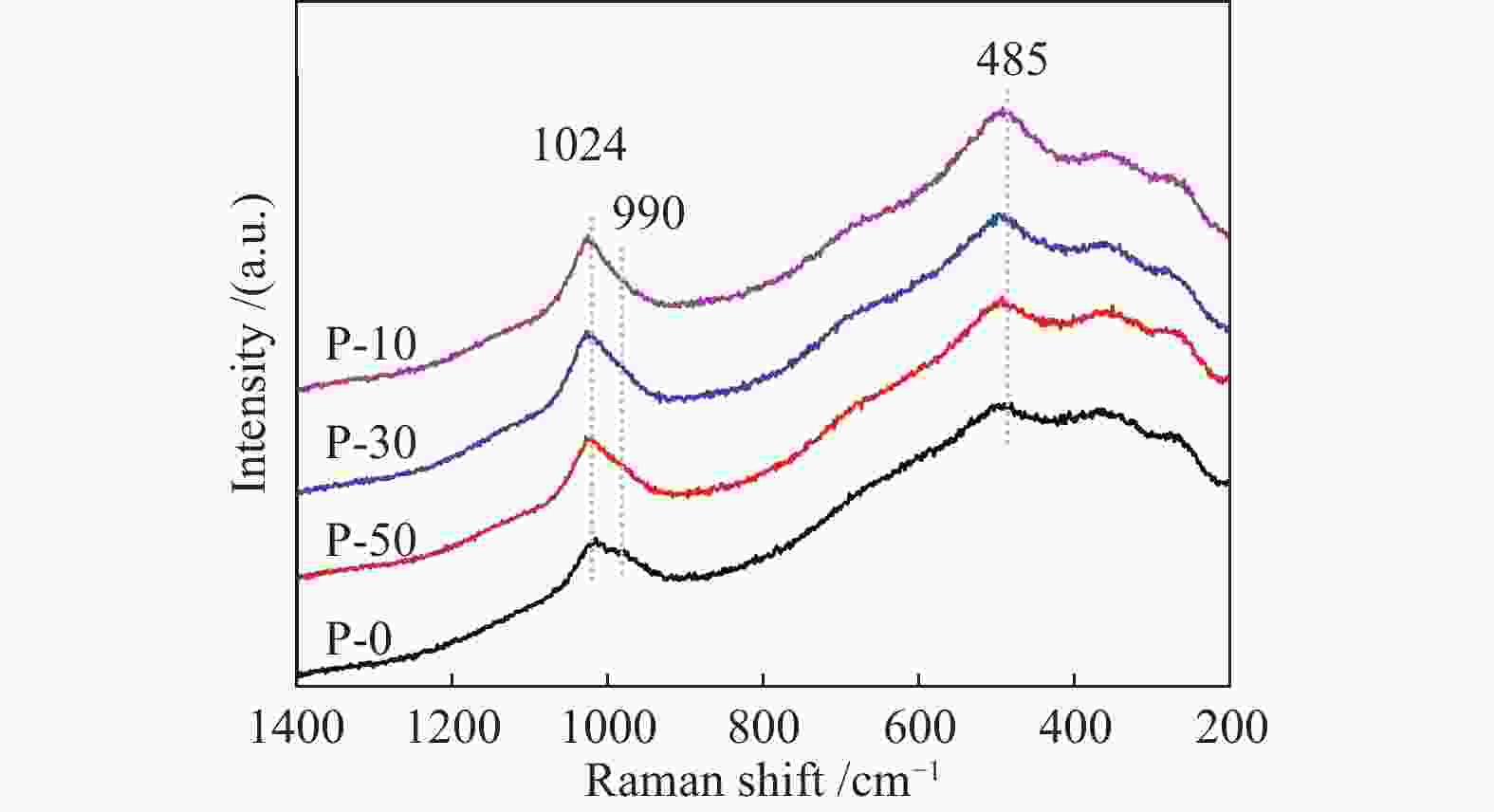
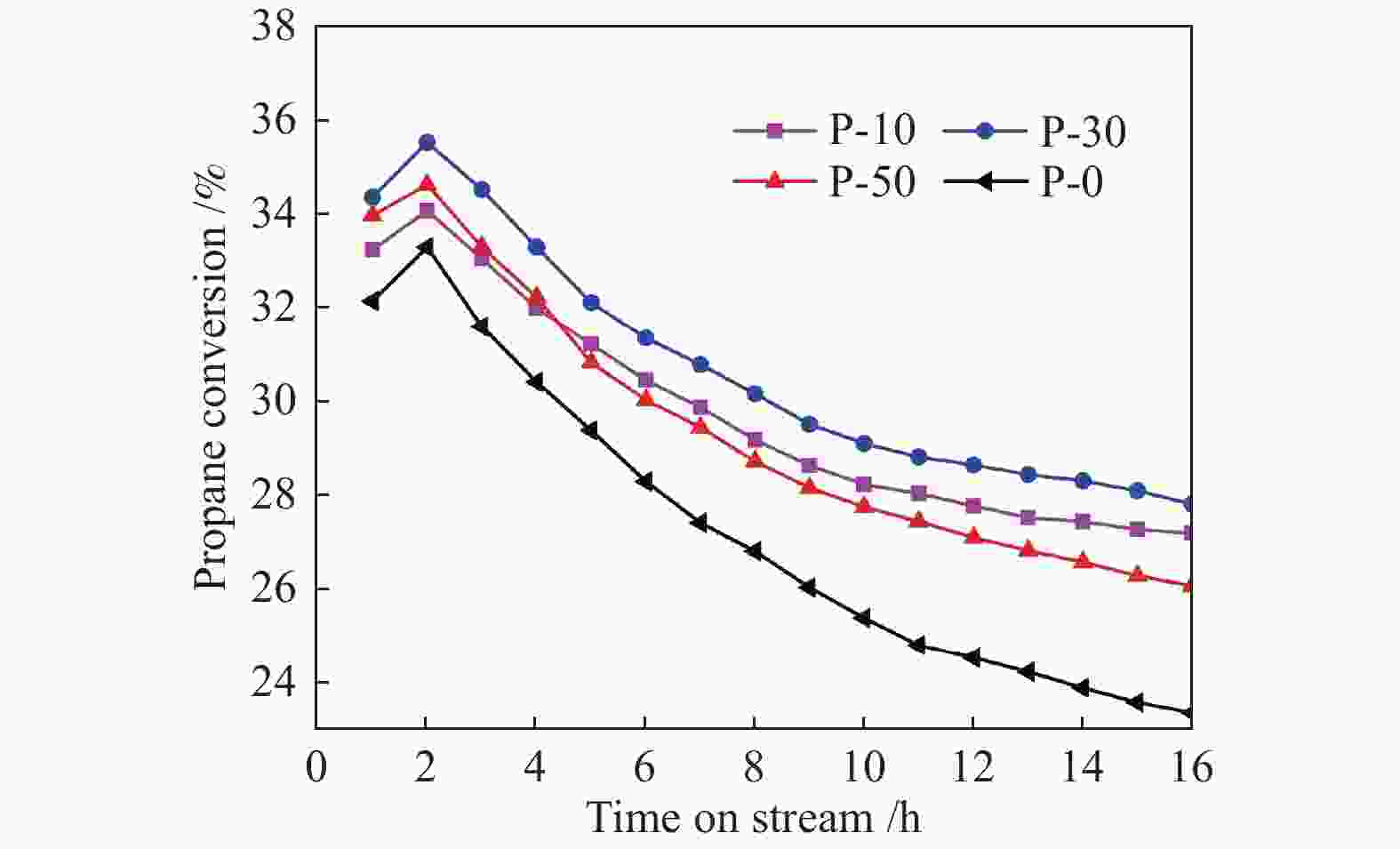
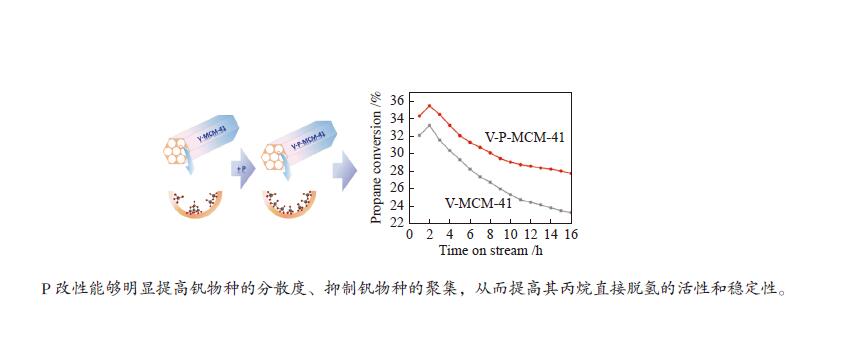
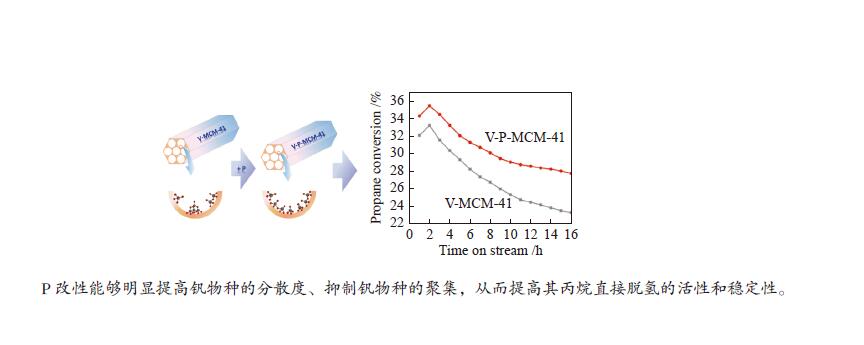
摘要: 钒基催化剂的脱氢性能与表面氧钒物种的形态密切相关。为了进一步增强传统原位合成的V-MCM-41催化剂上钒物种的分散性,本研究通过在制备过程中添加有机磷前驱物的方法对其进行改性。采用XRD、N2吸附-脱附、TPR、TPD、XPS、拉曼光谱及O2脉冲等方法对催化剂的结构、钒物种形态及分散度进行了系统的表征。表征结果表明,P改性后V-MCM-41催化剂的比表面积随着P含量的增加而缓慢下降,但整体仍能保持有序的六方介孔结构;P改性后表面钒物种的还原性和分散性均得到改善,聚合形态的钒物种比例明显下降。丙烷脱氢反应结果表明P改性后催化剂的丙烷脱氢性能和稳定性均有提高。在 Si/P 投料物质的量比为 30 时制备的催化剂能够获得最大表面钒氧位点和最佳丙烷脱氢性能。Abstract: The dehydrogenation performance of vanadyl catalysts was closely related to the form of surface vanadyl species. To enhance the vanadium dispersion, phosphorus was adopted to modify V-MCM-41 catalysts by using organic vanadium and phosphorus precursors. The influence of phosphorus introduction to the mesoporous structure and vanadyl species were investigated by various characterization techniques. The results showed that the catalysts could maintain ordered hexagonal mesoporous structures though the specific surface area slowly decreased along with the increase of phosphorus content. Both the reducibility and dispersion of the surface vanadyl species were improved. The proportion of polymerized vanadyl species obviously decreased due to the presence of phosphorus species. The propane dehydrogenation reaction results showed that both the catalytic performance and the catalyst stability were improved. Both the maximum surface vanadyl site density and optimum propane dehydrogenation performance were obtained over the sample with Si/P molar ratio of 30.
-
Key words:
- phosphorous modification /
- MCM-41 /
- vanadyl species /
- propane /
- dehydrogenation
-
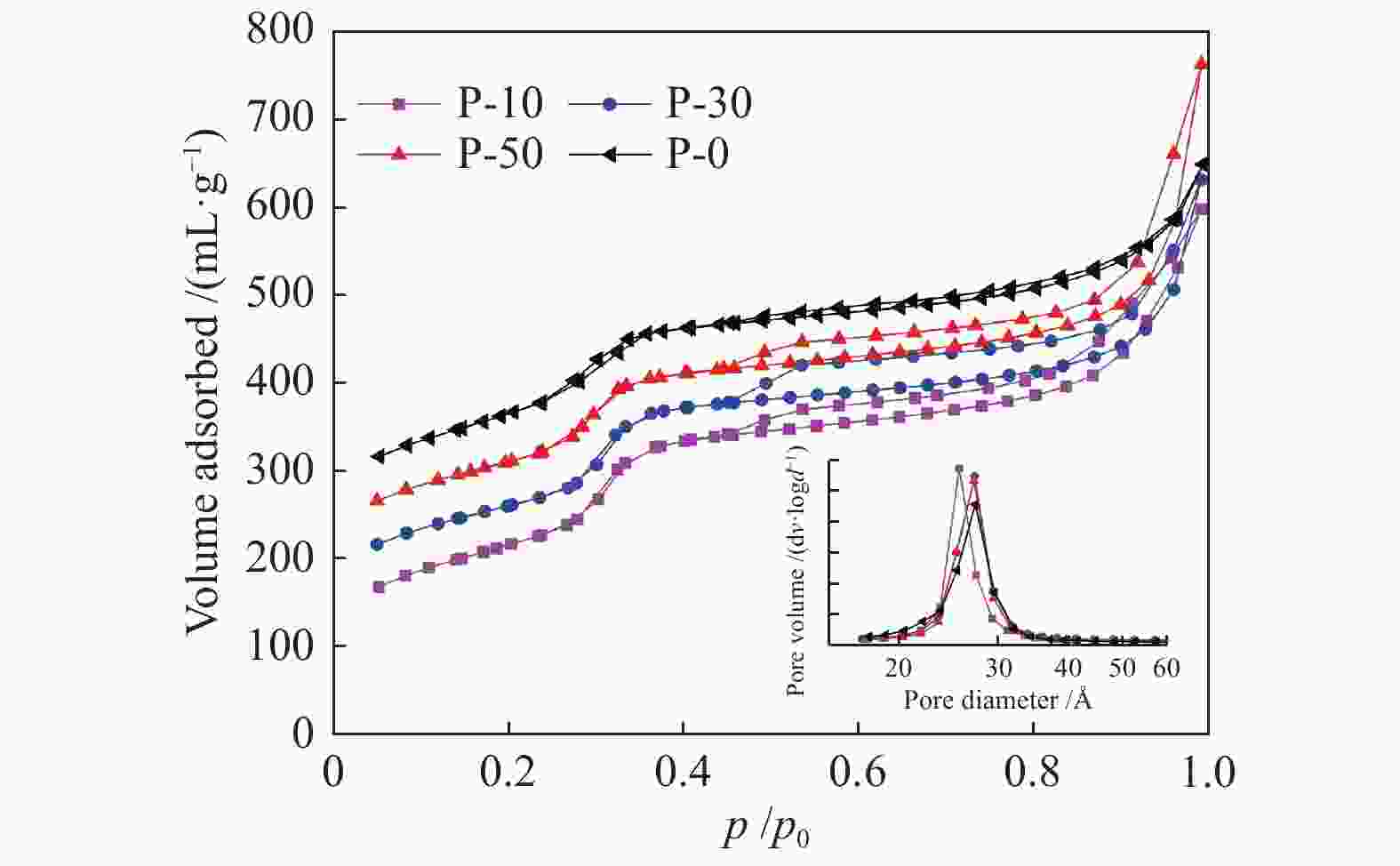
Table 1 Physio-chemical properties of V-P-MCM-41 catalysts
Sample SBET / (m2∙g−1) Pore volume /(mL∙g−1) d100 a /Å α b /Å r c /Å P-10 596.6 1.07 40.3 46.5 25.6 P-30 601.5 0.85 40.2 46.4 27.1 P-50 628.3 0.96 39. 9 46.1 27.0 P-0 668.6 0.73 39.0 45.1 27.6 a: [100] Crystalline interplanar spacing, calculated by Prague equation
b: Cell parameter, α=2d100/30.5
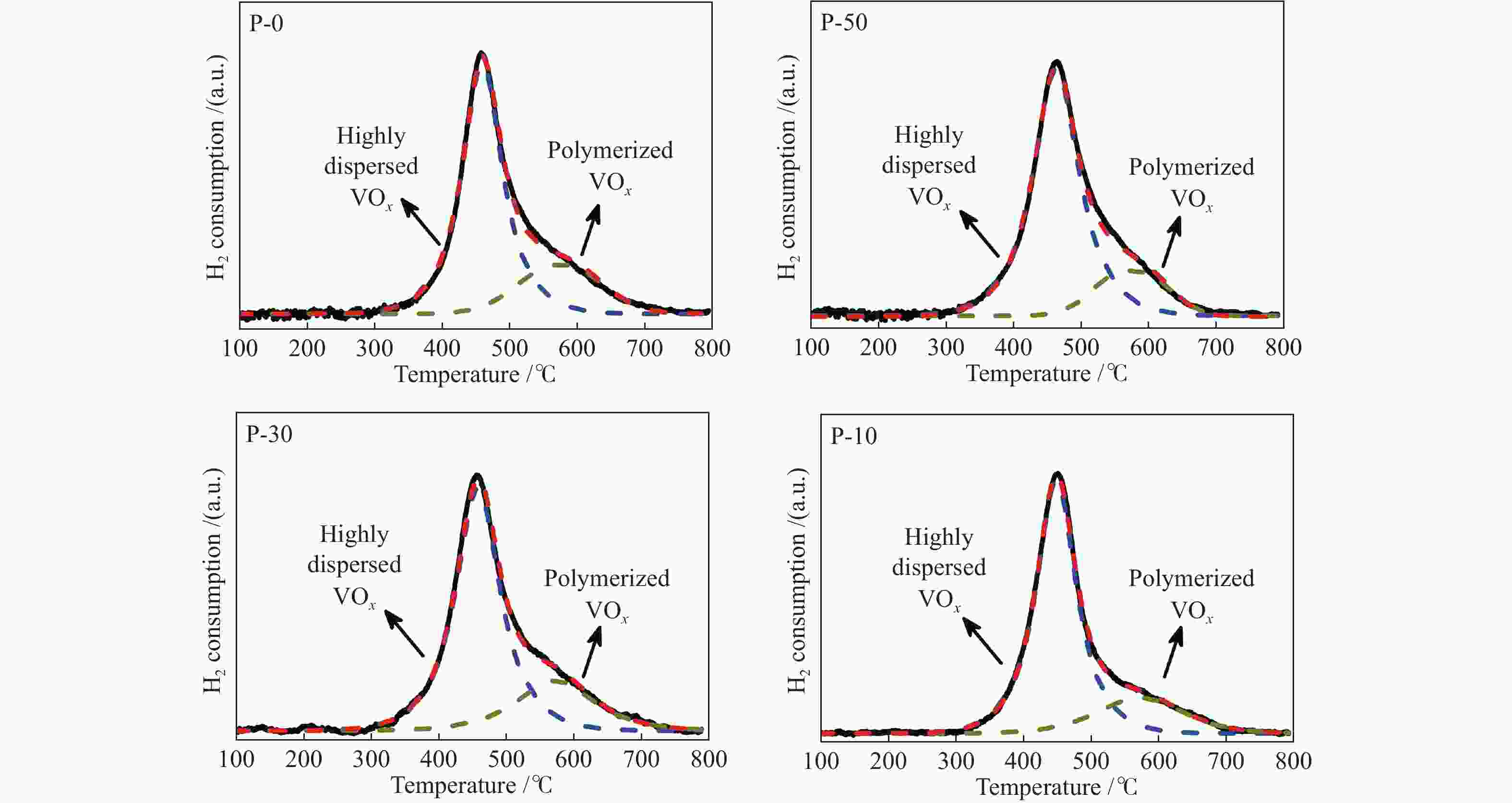
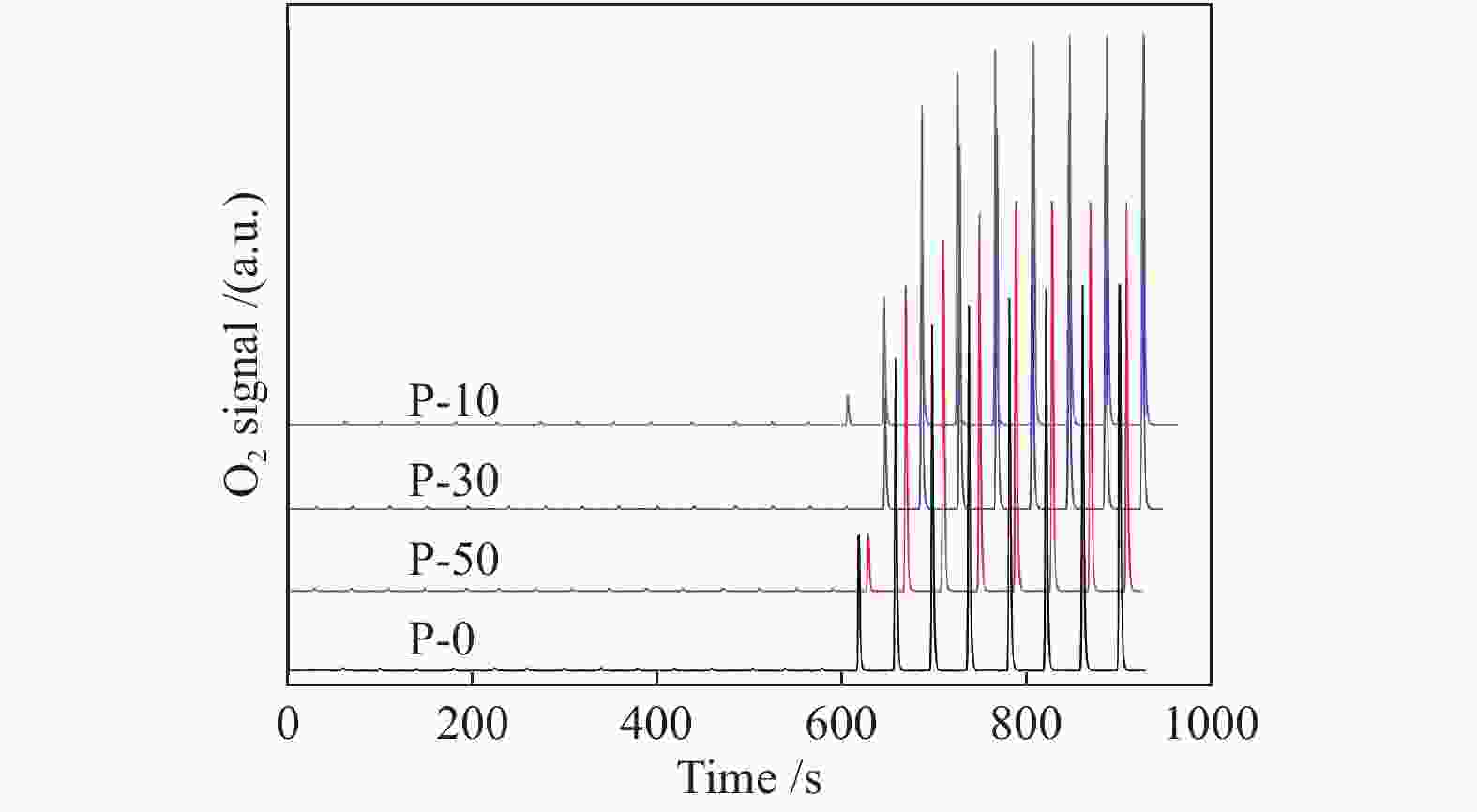
c: Pore diameterTable 2 TPR, TPD and O2 chemisorption results of V-P-MCM-41 catalysts
Sample Va/% Proportion of surface vanadyl species b Surface V sites c/
(10−4 mol∙g−1)Surface V density d/
nm−2highly dispersed polymerized P-10 3.49 80.5 19.5 3.19 3.21 P-30 3.45 78.8 21.9 3.35 3.35 P-50 3.43 75.0 25.0 3.29 3.15 P-0 3.38 74.3 25.7 3.11 2.80 a: measured by ICP-AES
b: determined by the deconvolution results of TPR
c: determined by O2 chemisorption
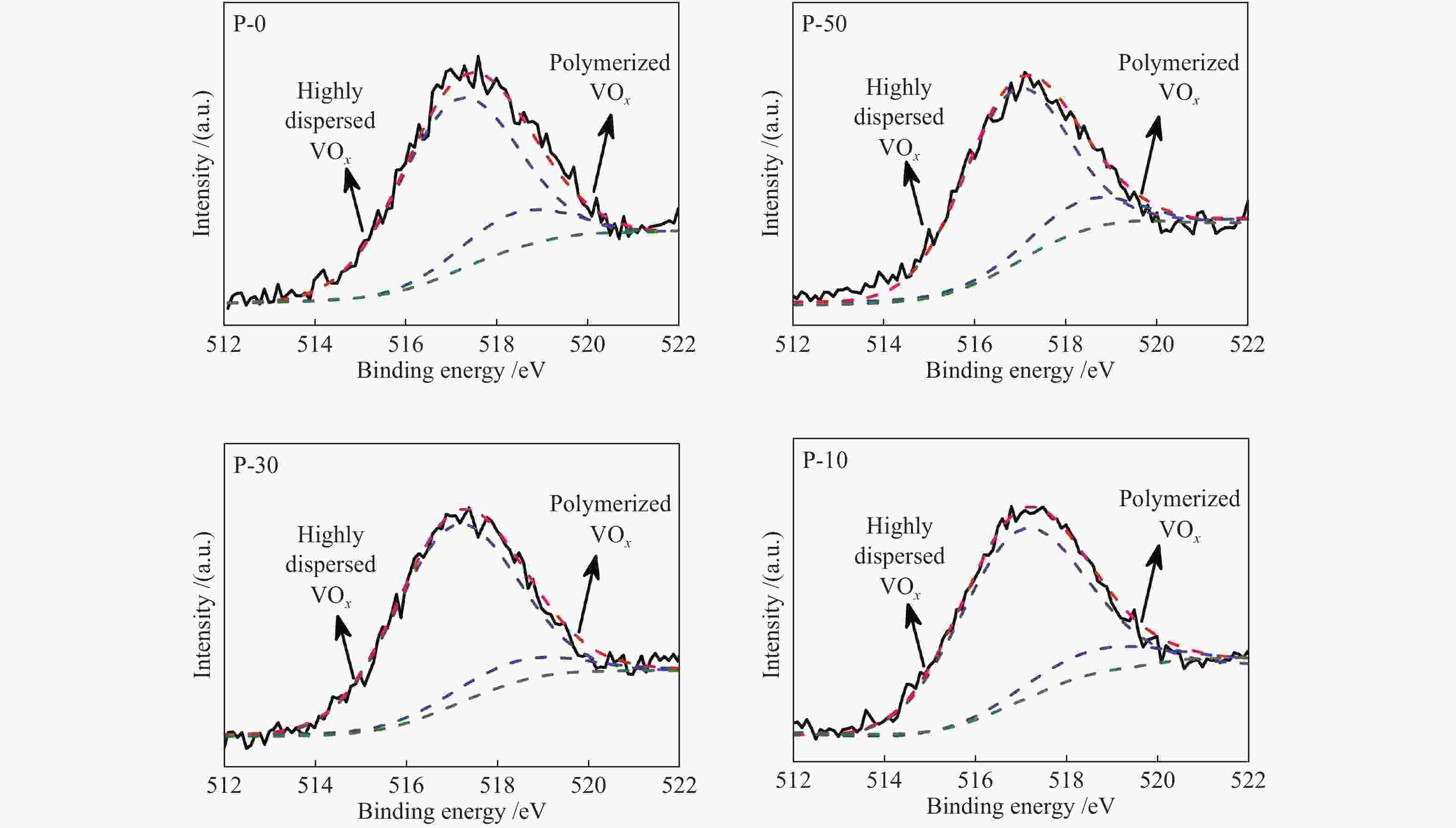
d: based on the surface V sites determined by O2 chemisorption and BET surface areaTable 3 XPS results of V-P-MCM-41
Sample E/eV Vanadyl species distribution / % O 1s Si 2p 517.0 eV 518.4 eV P-10 532.6 103.3 90.4 9.6 P-30 532.6 103.5 92.1 7.9 P-50 532.6 103.4 88.4 11.6 P-0 532.7 103.5 83.8 16.2 Table 4 Surface composition of V-P-MCM-41 catalysts determined by XPS
Sample Surface composition /mol % Surface V content O Si P V w/% P-10 63.42 32.81 2.48 1.28 3.12 P-30 65.12 32.45 1.14 1.29 3.17 P-50 64.64 33.21 0.87 1.28 3.16 P-0 65.22 33.47 − 1.31 3.03 -
[1] CAVANI F, BALLARINI N, CERICOLA A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation?[J]. Catal Today,2007,127(1/4):113−131. doi: 10.1016/j.cattod.2007.05.009 [2] GARTNER C A, VAN VEEN A, LERCHER J A. Oxidative dehydrogenation of ethane: Common principles and mechanistic Aspects[J]. ChemCatChem,2013,5(11):3196−3217. doi: 10.1002/cctc.201200966 [3] CARRERO C A, SCHLOEGL R, WACHS I E, SCHOMAECKER R. Critical literature review of the kinetics for the oxidative dehydrogenation of propane over well-defined supported vanadium oxide catalysts[J]. ACS Catal,2014,4(10):3357−3380. doi: 10.1021/cs5003417 [4] JAMES O O, MANDA S, ALELE N, CHOWDHURY B, MAITY S. Lower alkanes dehydrogenation: Strategies and reaction routes to corresponding alkenes[J]. Fuel Process Technol,2016,149:239−255. doi: 10.1016/j.fuproc.2016.04.016 [5] LONG L L, XIA K, LANG W Z, SHEN L L, YANG Q, YAN X, GUO Y J. The comparison and optimization of zirconia, alumina, and zirconia-alumina supported PtSnIn trimetallic catalysts for propane dehydrogenation reaction[J]. J Ind Eng Chem,2017,51:271−280. doi: 10.1016/j.jiec.2017.03.012 [6] SOKOLOV S, BYCHKOV V Y, STOYANOVA M, RODEMERCK U, BENTRUP U, LINKE D, TYULENIN Y P, KORCHAK V N, KONDRATENKO E V. Effect of VOx species and support on coke formation and catalyst stability in nonoxidative propane dehydrogenation[J]. ChemCatChem,2015,7(11):1691−1700. [7] WACHS I E. Catalysis science of supported vanadium oxide catalysts[J]. Dalton Trans,2013,42:11762−11769. doi: 10.1039/c3dt50692d [8] DONG A H, WANG K, ZHU S Z, YANG G B, WANG X T. Facile preparation of PtSn-La/Al2O3 catalyst with large pore size and its improved catalytic performance for isobutane dehydrogenation[J]. Fuel Process Technol,2017,158:218−225. doi: 10.1016/j.fuproc.2017.01.004 [9] HARLIN M E, NIEMI V M, KRAUSE A O. Alumina-supported vanadium oxide in the dehydrogenation of butanes[J]. J Catal,2000,195(1):67−78. doi: 10.1006/jcat.2000.2969 [10] LIU Y M, CA Y, YI N, FENG W L, DAI W L, YAN S R, HE H Y, FAN K N. Vanadium oxide supported on mesoporous SBA-15 as highly selective catalysts in the oxidative dehydrogenation of propane[J]. J Catal,2004,224(2):417−428. doi: 10.1016/j.jcat.2004.03.010 [11] REDDY B M, LAKSHMANAN P, LORIDANT S, YAMADA Y, KOBAYASHI T, LÓPEZ-CARTES C, ROJAS T C, FERNÁNDEZ A. Structural characterization and oxidative dehydrogenation activity of V2O5/CexZr1-xO2/SiO2 catalysts[J]. J Phys Chem B,2006,110(18):9140−9147. doi: 10.1021/jp061018k [12] RAJU G, REDDY B M, PARK S E. CO2 promoted oxidative dehydrogenation of n-butane over VOx/MO2-ZrO2 (M=Ce or Ti) catalysts[J]. J CO2 Util,2014,5:41−46. doi: 10.1016/j.jcou.2013.12.003 [13] ZHOU R, CAO Y, YAN S R, FAN K N. Rare earth (Y, La, Ce)-promoted V-HMS mesoporous catalysts for oxidative dehydrogenation of propane[J]. Appl Catal A: Gen,2002,236(1/2):103−111. [14] SASIKALA R, SUDARSAN V, KULSHRESHTHA S K. Studies on the interaction of vanadia with modified silica supports and catalytic activity for oxidative dehydrogenation of propane: Effect of support modification by Al3+, Zr4+ or Y3+[J]. Eur J Inorg Chem,2006,2006(20):4151−4156. [15] YANG S, IGLESIA E, BELL A T. Oxidative Dehydrogenation of Propane over V2O5/MoO3/Al2O3 and V2O5/Cr2O3/Al2O3: Structural characterization and catalytic function[J]. J Phys Chem B,2005,109(18):8987−9000. doi: 10.1021/jp040708q [16] AJAYI B P, JERMY B R, OGUNRONBI K E, ABUSSAUD B A, AL-KHATTAF S. n-Butane dehydrogenation over mono and bimetallic MCM-41 catalysts under oxygen free atmosphere[J]. Catal Today,2013,204:189−196. doi: 10.1016/j.cattod.2012.07.013 [17] ASCOOP I, GALVITA V V, ALEXOPOULOS K, REYNIERS M F, VAN DER VOORT P, BLIZNUK V, MARIN G B. The role of CO2 in the dehydrogenation of propane over WOx-VOx/SiO2[J]. J Catal,2016,335:1−10. doi: 10.1016/j.jcat.2015.12.015 [18] OYAMA S T, WENT G T, LEWIS K B, BELL A T, SOMORJAI G A. Oxygen-chemisorption and Laser Raman-spectroscopy of unsupported and silica-supported vanadium-oxide catalysts[J]. J Phys Chem,1989,93(18):6786−6790. [19] WANG X, ZHOU G, CHEN Z, LI Q, ZHOU H, XU C. Enhancing the vanadium dispersion on V-MCM-41 by boron modification for efficient iso-butane dehydrogenation[J]. Appl Catal A: Gen,2018,555:171−177. doi: 10.1016/j.apcata.2018.02.021 [20] SHYLESH S, SINGH A. Vanadium-containing ordered mesoporous silicates: Does the silica source really affect the catalytic activity, structural stability, and nature of vanadium sites in V-MCM-41[J]? J Catal, 2005, 233(2): 359−371. [21] WANG C B, DEO G, WACHS I E. Characterization of vanadia sites in V-silicalite, vanadia-silica cogel, and silica-supported Vanadia catalysts: X-ray powder diffraction, Raman Spectroscopy, Solid-State 51V NMR, Temperature-Programmed Reduction, and Methanol Oxidation Studies[J]. J Catal, 1998,1998,178(2):640−648. [22] SOLSONA B, BLASCO T, LÓPEZ NIETO J M, PEñA M L, REY F, VIDAL-MOYA A. Vanadium oxide supported on mesoporous MCM-41 as selective catalysts in the oxidative dehydrogenation of alkanes[J]. J Catal,2001,203(2):443−452. [23] WANG X, ZHOU G, CHEN Z, JIANG W, ZHOU H. In-situ synthesis and characterization of V-MCM-41 for oxidative dehydrogenation of n-butane[J]. Microporous Mesoporous Mater,2016,223:261−267. [24] LIU Q L, YANG Z, LUO M S, ZHAO Z, WANG J Y, XIE Z A, GUO L. Vanadium-containing dendritic mesoporous silica nanoparticles: Multifunctional catalysts for the oxidative and non-oxidative dehydrogenation of propane to propylene[J]. Microporous Mesoporous Mater,2019,282:133−145. [25] LI X K, JI W J, ZHAO J, ZHANG Z, AU C T. A comparison study on the partial oxidation of n-butane and propane over VPO catalysts supported on SBA-15, MCM-41, and fumed SiO2[J]. Appl Catal A: Gen, 2006, 306: 8−16. [26] ARIAS-PÉREZ S, GARCÍA-ALAMILLA R, CÁRDENAS-GALINDO M G, HANDY B E, ROBLES-ANDRADE S, SANDOVAL-ROBLES G. Evaluation of vanadium-phosphorus oxide (VPO) catalysts for the oxidative dehydrogenation of propane[J]. Ind Eng Chem Res,2009,48(3):1215−1219. [27] BULÁNEK R, KALUŽOVÁA, SETNIČKA M, ZUKAL A, ČIČMANEC P, MAYEROVÁ J. Study of vanadium based mesoporous silicas for oxidative dehydrogenation of propane and n-butane[J]. Catal Today,2012,179(1):149−158. [28] SETNIČKA M, BULÁNEK R, ČAPEK L, ČIČMANEC P. n-Butane oxidative dehydrogenation over VOx-HMS catalyst[J]. J Mol Catal A: Chem,2011,344(1/2):1−10. [29] SANTRA C, SHAH S, MONDAL A, PANDEY J K, PANDA A B, MAITY S, CHOWDHURY B. Synthesis, characterization of VPO catalyst dispersed on mesoporous silica surface and catalytic activity for cyclohexane oxidation reaction[J]. Microporous Mesoporous Mater,2016,223:121−128. [30] PUTLURU S, RIISAGER A, FEHRMANN R. The effect of acidic and redox properties of V2O5/CeO2-ZrO2 catalysts in selective catalytic reduction of NO by NH3[J]. Catal Lett,2009,133:370−375. [31] HU P, LANG W Z, YAN X, CHU L F, GUO Y J. Influence of gelation and calcination temperature on the structure-performance of porous VOx-SiO2 solids in non-oxidative propane dehydrogenation[J]. J Catal,2018,358:108−117. [32] ROSTOM S, DE LASA H I. Propane oxidative dehydrogenation using consecutive feed injections and fluidizable VOx/gamma Al2O3 and VOx/ZrO2-gamma Al2O3 catalysts[J]. Ind Eng Chem Res,2017,56:13110−13125. [33] WACHS I E, WECKHUYSEN B M. Vanadia catalysts for selective oxidation of hydrocarbons and their derivatives structure and reactivity of surface vanadium oxide species on oxide supports[J]. Appl Catal A: Gen,1997,157(1/2):67−90. [34] CARRERO C A, KETURAKIS C J, ORREGO A, SCHOMACKER R, WACHS I E. Anomalous reactivity of supported V2O5 nanoparticles for propane oxidative dehydrogenation: Influence of the vanadium oxide precursor[J]. Dalton Trans,2013,42:12644−12653. [35] MURGIA V, TORRES E, GOTTIFREDI J, SHAM E. Sol-gel synthesis of V2O5-SiO2 catalyst in the oxidative dehydrogenation of n-butane[J]. Appl Catal A: Gen,2006,312:134−143. [36] ARENA F, FRUSTERI F, MARTRA G, COLUCCIA S, PARMALIANA A. Surface structures, reduction pattern and oxygen chemisorption of V2O5/SiO2catalysts[J]. J Chem Soc Faraday Trans,1997,93:3849−3854. [37] FUKUDOME K, IKENAGA N, MIYAKE T, SUZUKI T. Oxidative dehydrogenation of propane using lattice oxygen of vanadium oxides on silica[J]. Catal Sci Technol,2011,1:987−998. [38] BAI P, MA Z, LI T, TIAN Y, ZHANG Z, ZHONG Z, XING W, WU P, LIU X, YAN Z. Relationship between surface chemistry and catalytic performance of mesoporous γ-Al2O3 supported VOx catalyst in catalytic dehydrogenation of propane[J]. ACS Appl Mater Interfaces,2016,8(39):25979−25990. [39] TIAN Y P, BAI P, LIU S M, LIU X M, YAN Z F. VOx-K2O/γ-Al2O3 catalyst for nonoxidative dehydrogenation of isobutane[J]. Fuel Process Technol,2016,151:31−39. [40] CHEN C, SUN M L, HU Z P, LIU Y P, ZHANG S M, YUAN Z Y. Nature of active phase of VOx catalysts supported on SiBeta for direct dehydrogenation of propane propylene[J]. Chin J Catal,2020,41(2):276−285. [41] CASALETTO M P, LISI L, MATTOGNO G, PATRONO P, RUOPPOLO G. An XPS study of titania-supported vanadyl phosphate catalysts for the oxidative dehydrogenation of ethane[J]. Appl Catal A: Gen,2004,267(1/2):157−164. [42] HASHA D, SIERRA DE SALDARRIAGA L, SALDARRIAGA C, HATHAWAY P E, COX D F, DAVIS M E. Studies of silicoaluminophosphates with the sodalite structure[J]. J Am Chem Soc,1988,110:2127−2135. [43] EBERHARDT M A, PROCTOR A, HOUALLA M, HERCULES D M. Investigation of V oxidation states in reduced V/Al2O3 catalysts by XPS[J]. J Catal,1996,160(1):27−34. [44] GAO X, JEHNG J M, WACHS I E. In situ UV-vis-NIR diffuse reflectance and Raman spectroscopic studies of propane oxidation over ZrO2-supported vanadium oxide catalysts[J]. J Catal,2002,209(1):43−50. [45] RESINI C, MONTANARI T, BUSCA G, JEHNG J M, WACHS I E. Comparison of alcohol and alkane oxidative dehydrogenation reactions over supported vanadium oxide catalysts: in situ infrared, Raman and UV-vis spectroscopic studies of surface alkoxide intermediates and of their surface chemistry[J]. Catal Today,2005,99(1/2):105−114. [46] DZWIGAJ S. Recent advances in the incorporation and identification of vanadium species in microporous materials[J]. Curr Opin Solid State Mater Sci,2003,7(6):461−470. [47] PIUMETTI M, BONELLI B, MASSIANI P, DZWIGAJ S, ROSSETTI I, CASALE S, GABEROVA L, ARMANDI M, GARRONE E. Effect of vanadium dispersion and support properties on the catalytic activity of V-SBA-15 and V-MCF mesoporous materials prepared by direct synthesis[J]. Catal Today,2011,176(1):458−464. [48] CHRISTODOULAKIS A. Molecular structure and reactivity of vanadia-based catalysts for propane oxidative dehydrogenation studied by in situ Raman spectroscopy and catalytic activity measurements[J]. J Catal,2004,222(2):293−306. [49] RODEMERCK U, SOKOLOV S, STOYANOVA M, BENTRUP U, LINKE D, KONDRATENKO E V. Influence of support and kind of VOx species on isobutene selectivity and coke deposition in non-oxidative dehydrogenation of isobutane[J]. J Catal,2016,338:174−183. [50] OVSITSER O, SCHOMAECKER R, KONDRATENKO E V, WOLFRAM T, TRUNSCHKE A. Highly selective and stable propane dehydrogenation to propene over dispersed VOx-species under oxygen-free and oxygen-lean conditions[J]. Catal Today,2012,192(1):16−19. -




 下载:
下载: