Effect of iron-based catalyst from coal liquefaction on coal char gasification reactivity and kinetics
-
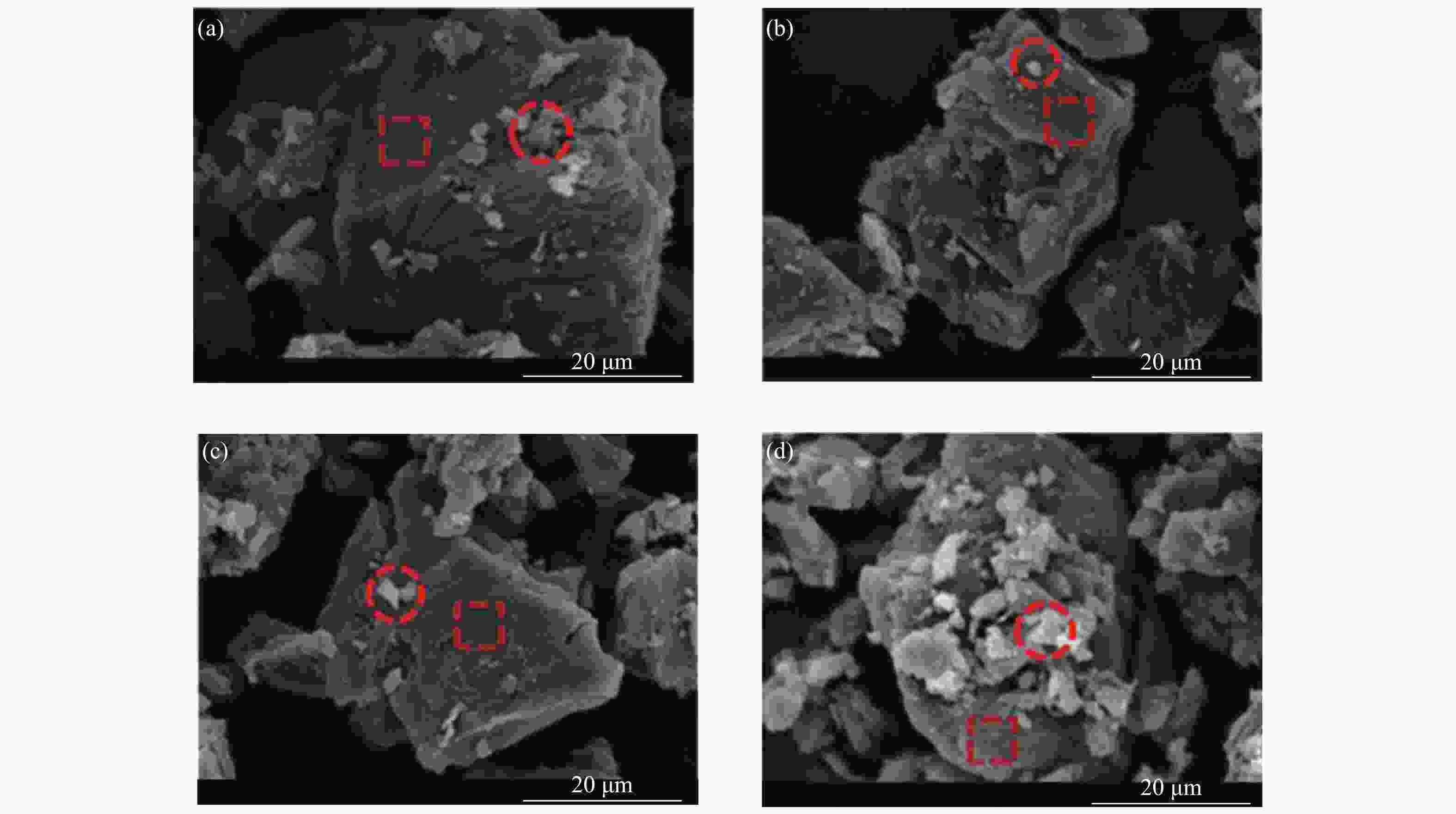
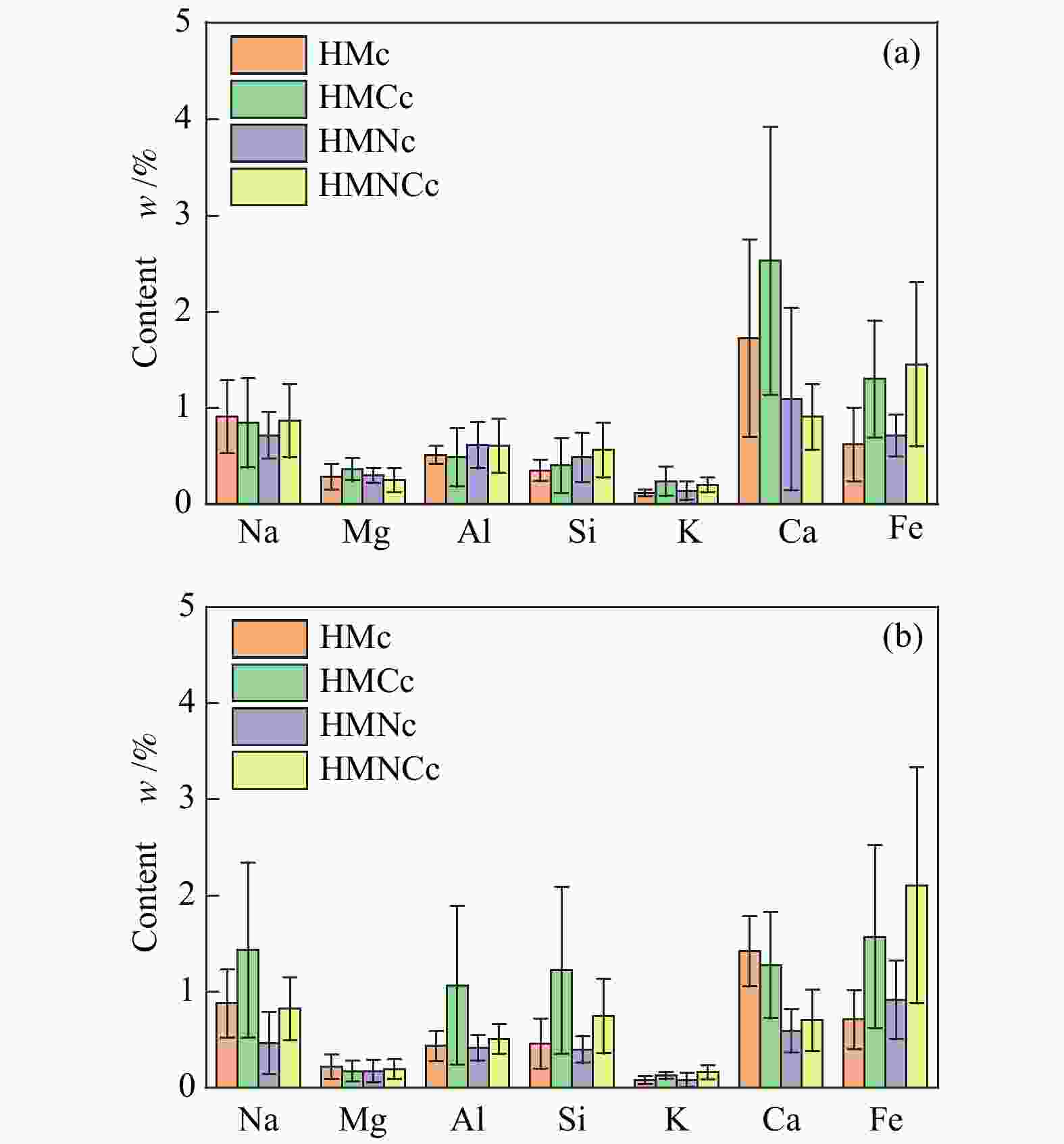
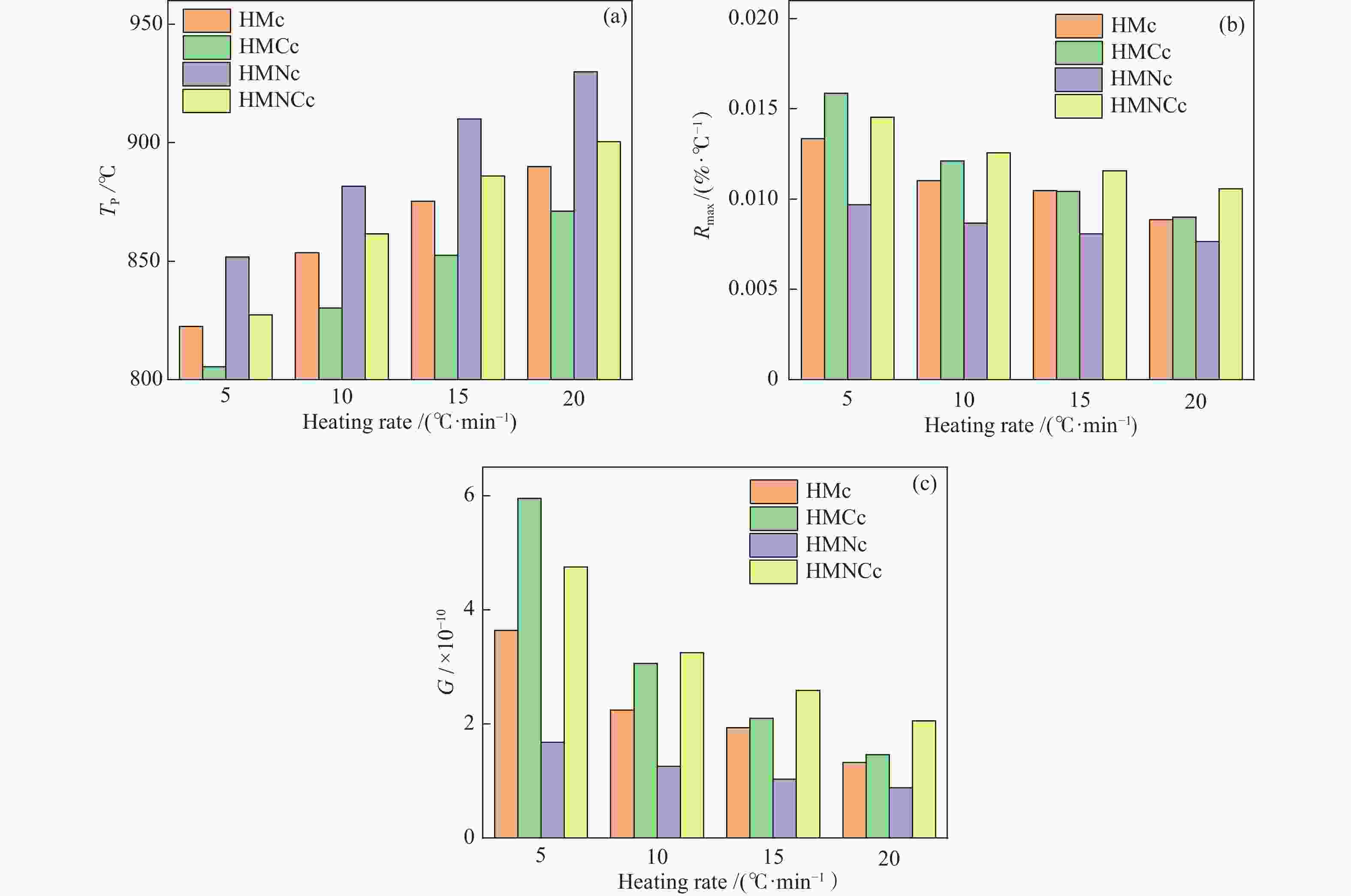
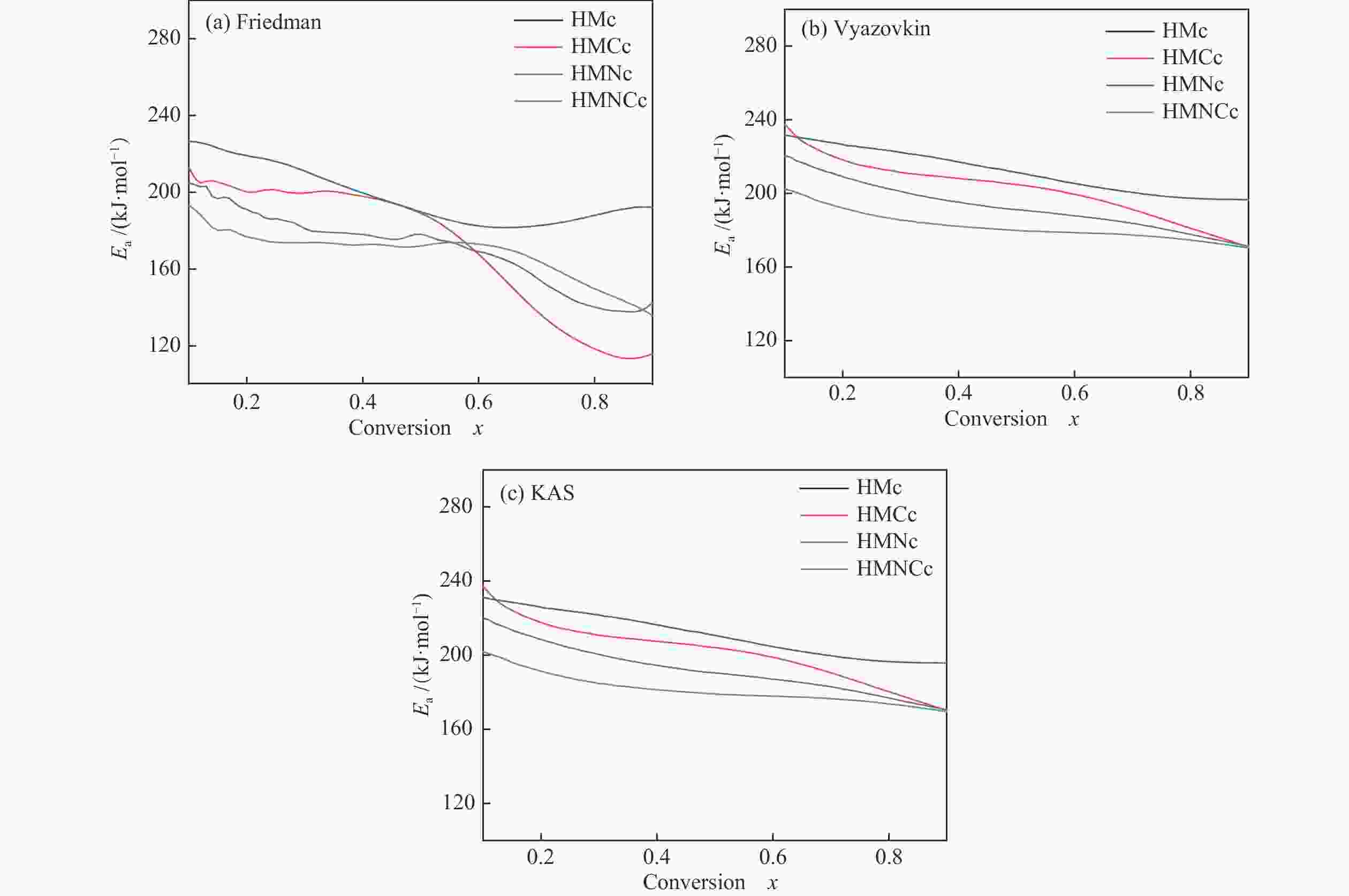
摘要: 本研究以哈密原煤和脱矿煤为原料,研究煤液化铁基催化剂对煤焦结构和气化反应性的影响。利用扫描电镜能谱仪和物理吸附仪研究煤焦表面形貌、元素分布和介孔特性,利用热重分析仪研究煤焦气化特性,采用model-fitting和model-free方法研究反应动力学。结果表明,脱矿和负载催化剂对煤焦表面附着物的影响较主体基质明显。负载催化剂的煤焦比表面积显著增加。铁基催化剂提升煤焦气化活性可归因于煤焦表面富集较多Fe和AAEMs等元素,以及比表面积的增大。负载催化剂的脱矿煤焦表现出较大的相对催化活性,且其对升温速率和碳转化率的变化不敏感。煤焦气化特性的差异将随升温速率的升高而减小。铁基催化剂可提高原煤焦的气化反应指前因子A,降低脱矿煤焦的反应活化能Ea。非等温条件下,煤焦气化反应活化能随转化率的增加而降低。根据模型拟合度和动力学补偿效应,随机孔模型是描述煤焦气化的最佳模型,且更适合于脱矿煤焦(催化)气化。Abstract: In the present work, the effects of iron-based catalysts from coal liquefaction on the coal structure and gasification reactivity were studied using the Hami raw coal and demineralized coal. The surface morphology, element distribution and mesoporous characteristics of coal char were investigated by SEM-EDS and physical adsorption analyzer. The gasification reactivity was performed in a thermogravimetric analyzer. The gasification kinetics was studied through the model-fitting and model-free methods. The results showed that the demineralization and catalyst loading had more obvious effect on the surface attachments than the carbon matrix. The coal char with catalyst loading had significant larger specific surface area (SSA). The reactivity improvement by iron-based catalyst was attributed to the enrichment of Fe and AAEMS and increase of SSA for coal char. More pronounced relative catalytic activity was observed for the catalytic gasification of demineralized coal char, and its activity was not sensitive to the change of heating rate and carbon conversion. The gasification characteristics difference decreased with the increase of heating rate. The iron-based catalyst increased the pre-exponential factor A for the demineralized coal char gasification, and reduced the activation energy Ea for the raw coal char gasification. Under the non-isothermal conditions, the Ea decreased with conversion. According to fitting performance and kinetic compensation effect, the random pore model was the best model to describe gasification, especially for the (catalytic) gasification of the demineralized coal char.
-
Key words:
- coal gasification /
- kinetics /
- Fe-based catalyst /
- catalytic gasification /
- demineralization
-
表 1 HM煤的工业分析和元素分析
Table 1 Proximate and ultimate analyses of HM coal
Sample Proximate analysis wd/% Ultimate analysis wdaf/% V A FC C H O* N S HM 48.76 7.23 44.02 65.65 5.93 19.66 0.83 0.70 HMc 12.12 10.84 77.04 83.95 1.10 1.91 1.24 0.96 HMCc 12.84 17.56 69.60 78.54 1.15 0.51 1.19 1.04 HMNc 6.69 9.11 84.20 86.75 1.19 0.65 1.38 0.92 HMNCc 5.67 13.65 80.68 82.46 1.13 0.23 1.15 1.38 V: volatile; A: ash; FC: fixed carbon; d: dry basis; daf: dry ash-free basis; *: by difference 表 2 HM煤灰分组成
Table 2 Ash composition of HM coal
Contet w/% Residual CaO SO3 SiO2 Al2O3 Fe2O3 Na2O MgO P2O5 TiO2 K2O 26.89 16.80 16.06 15.46 14.28 5.32 2.97 0.40 0.30 0.27 1.25 表 3 煤焦介孔结构参数
Table 3 Mesopore parameters of coal char
Sample SSA/(m2·g−1) PV/(cm3·g−1) APD /nm HMc 0.10 0.003 43.3 HMCc 170.42 0.107 2.5 HMNc 0.27 0.001 18.5 HMNCc 96.54 0.062 2.5 SSA: specific surface area; PV: pore volume; APD: average pore distribution 表 4 Model-fitting分析动力学参数
Table 4 Kinetic parameters of model-fitting analysis (lnA: min−1, Ea: kJ/mol)
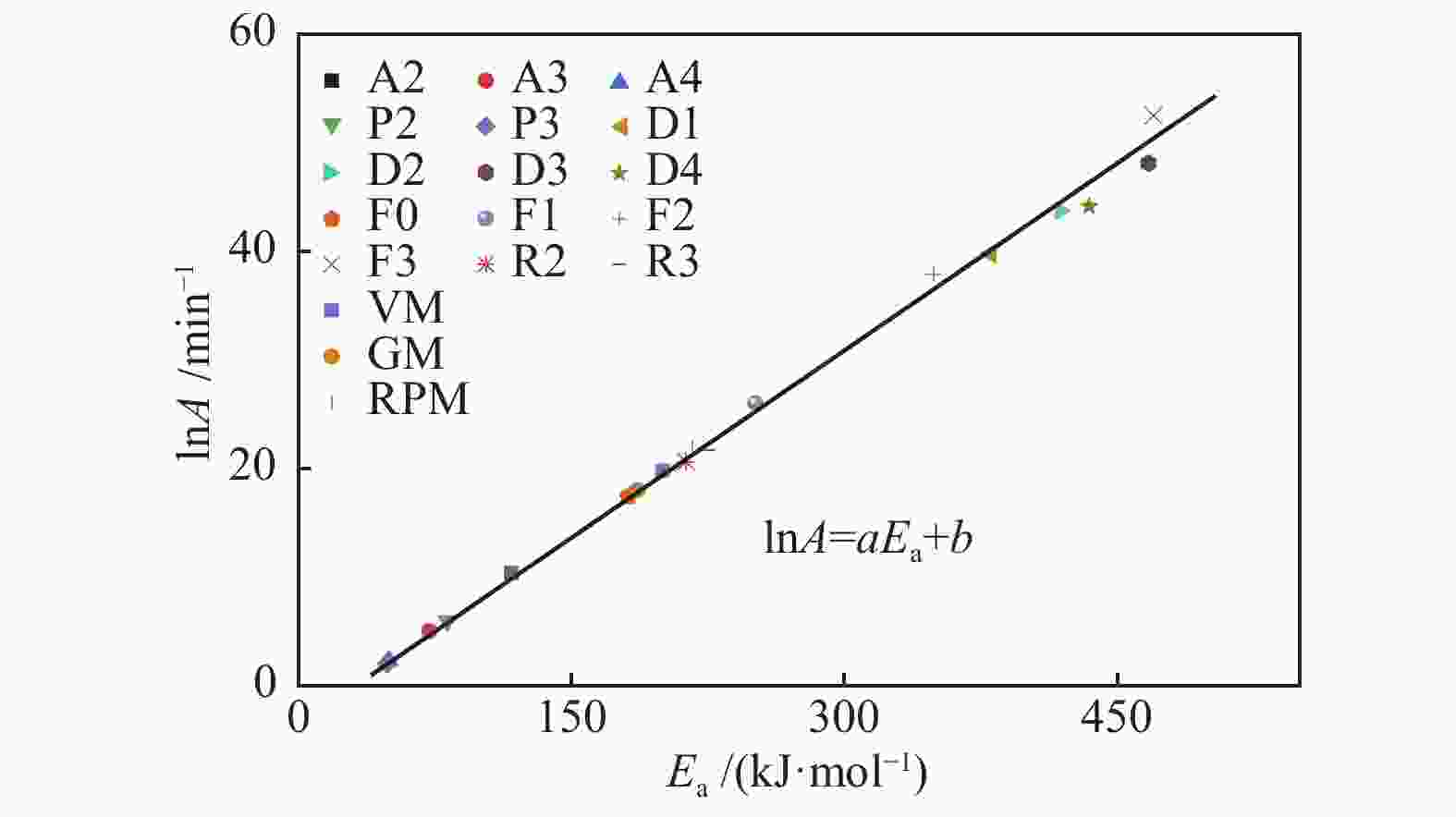
Sample VM GM RPM lnA Ea SSR lnA Ea SSR lnA Ea Ψ SSR HMc 21.9 216.6 0.566 19.8 200.0 0.213 18.0 186.6 4.17 0.092 HMCc 22.9 221.5 0.563 20.8 204.5 0.277 19.6 195.7 2.76 0.231 HMNc 21.4 219.8 0.111 20.1 210.0 0.059 19.6 205.3 0.83 0.031 HMNCc 20.9 208.7 0.797 19.1 194.0 0.355 16.9 178.1 5.81 0.156 表 5 Model-free和动力学补偿分析参数
Table 5 Kinetic parameters of model-free and kinetic compensation effect analysis (lnA: min−1, Ea: kJ/mol)
Sample Model-free analysis KCE analysis Friedman Vyazovkin KAS lnA Ea R2 HMc 171.82 193.45 192.71 17.39 182.11 0.998 HMCc 171.40 203.14 202.46 18.47 188.17 0.988 HMNc 198.92 212.22 211.50 19.28 206.82 0.999 HMNCc 167.68 182.55 181.78 16.81 177.73 0.999 -
[1] 王明华, 蒋文化, 韩一杰. 现代煤化工发展现状及问题分析[J]. 化工进展,2017,36(8):2882−2887.WANG Ming-hua, JIANG Wen-hua, HAN Yi-jie. Analysis on the present situation and problems of modern coal-chemical industry[J]. Chem Ind Eng Prog,2017,36(8):2882−2887. [2] CHU X, LI W, LI B, CHEN H. Sulfur transfers from pyrolysis and gasification of direct liquefaction residue of Shenhua coal[J]. Fuel,2008,87(2):211−215. doi: 10.1016/j.fuel.2007.04.014 [3] ZHANG X, SONG X, WANG J, SU W, BAI Y, ZHOU B, YU G. CO2 gasification of Yangchangwan coal catalyzed by iron-based waste catalyst from indirect coal-liquefaction plant[J]. Fuel,2021,285:119228. doi: 10.1016/j.fuel.2020.119228 [4] ZHAO D, LIU H, LU P, SUN B, GUO S, QIN M. DFT study of the catalytic effect of Fe on the gasification of char-CO2[J]. Fuel,2021,292. [5] LIU D, GAO J, WU S, QIN Y. Effect of char structures caused by varying the amount of FeCl3 on the pore development during activation[J]. RSC Adv,2016,6(90):87478−87485. doi: 10.1039/C6RA14712G [6] XU B, CAO Q, KUANG D, GASEM K A M, ADIDHARMA H, DING D, FAN M. Kinetics and mechanism of CO2 gasification of coal catalyzed by Na2CO3, FeCO3 and Na2CO3-FeCO3[J]. J Energy Inst,2020,93(3):922−933. doi: 10.1016/j.joei.2019.08.004 [7] ZHANG F, SUN H, BI J, QU X, YAN S, ZHANG J, ZHANG J. The evolution of Fe and Fe-Ca catalysts during char catalytic hydrogasification[J]. Fuel,2019,257:116040. doi: 10.1016/j.fuel.2019.116040 [8] LAHIJANI P, ZAINAL Z A, MOHAMED A R. Catalytic effect of iron species on CO2 gasification reactivity of oil palm shell char[J]. Thermochim Acta,2012,546:24−31. doi: 10.1016/j.tca.2012.07.023 [9] YU G, YU D, LIU F, YU X, HAN J, WU J, XU M. Different catalytic action of ion-exchanged calcium in steam and CO2 gasification and its effects on the evolution of char structure and reactivity[J]. Fuel,2019,254. [10] HE Q, YU J, SONG X, DING L, WEI J, YU G. Utilization of biomass ash for upgrading petroleum coke gasification: Effect of soluble and insoluble components[J]. Energy,2020,192:116642. doi: 10.1016/j.energy.2019.116642 [11] HE Q, GUO Q, UMEKI K, DING L, WANG F, YU G. Soot formation during biomass gasification: A critical review[J]. Renewable Sustainable,2021,139:110710. doi: 10.1016/j.rser.2021.110710 [12] 林善俊, 周志杰, 霍威, 丁路, 于广锁. 内扩散对煤和石油焦水蒸气气化反应性能的影响[J]. 燃料化学学报,2014,8:905−912. doi: 10.3969/j.issn.0253-2409.2014.08.002LIN Shanjun, ZHOU Zhijie, HUO Wei, DING Lu, YU Guangsuo. Effect of internal diffusion on steam gasification reactivity of coal and petroleum coke[J]. J Fuel Chem Technol,2014,8:905−912. doi: 10.3969/j.issn.0253-2409.2014.08.002 [13] ZHANG F, XU D, WANG Y, ARGYLE M D, FAN M. CO2 gasification of Powder River Basin coal catalyzed by a cost-effective and environmentally friendly iron catalyst[J]. Appl Energy,2015,145:295−305. doi: 10.1016/j.apenergy.2015.01.098 [14] MONTERROSO R, FAN M, ZHANG F, GAO Y, POPA T, ARGYLE M D, TOWLER B, SUN Q. Effects of an environmentally-friendly, inexpensive composite iron–sodium catalyst on coal gasification[J]. Fuel,2014,116:341−349. doi: 10.1016/j.fuel.2013.08.003 [15] HE Q, DING L, GONG Y, LI W, WEI J, YU G. Effect of torrefaction on pinewood pyrolysis kinetics and thermal behavior using thermogravimetric analysis[J]. Bioresour Technol,2019,280:104−111. doi: 10.1016/j.biortech.2019.01.138 [16] GUO Q, HUANG Y, HE Q, GONG Y, YU G. Analysis of coal gasification reactivity, kinetics, and mechanism with iron-based catalyst from coal liquefaction[J]. ACS Omega,2021,6(2):1584−1592. doi: 10.1021/acsomega.0c05425 [17] ELLIS N, MASNADI M S, ROBERTS D G, KOCHANEK M A, ILYUSHECHKIN A Y. Mineral matter interactions during co-pyrolysis of coal and biomass and their impact on intrinsic char co-gasification reactivity[J]. Chem Eng J,2015,279:402−408. doi: 10.1016/j.cej.2015.05.057 [18] LIANG D, XIE Q, ZHOU H, YANG M, CAO J, ZHANG J. Catalytic effect of alkali and alkaline earth metals in different occurrence modes in Zhundong coals[J]. Asia-Pac J Chem Eng,2018,13(3):e2190. doi: 10.1002/apj.2190 [19] HE Q, DING L, RAHEEM A, GUO Q, GONG Y, YU G. Kinetics comparison and insight into structure-performance correlation for leached biochar gasification[J]. Chem Eng J,2021,129331. [20] JAYARAMAN K, KOK M V, GOKALP I. Pyrolysis, combustion and gasification studies of different sized coal particles using TGA-MS[J]. Appl Therm Eng,2017,125:1446−1455. doi: 10.1016/j.applthermaleng.2017.07.128 [21] ZHAO M, RAHEEM A, MEMON Z M, VUPPALADADIYAM A K, JI G. Iso-conversional kinetics of low-lipid micro-algae gasification by air[J]. J Clean Prod,2019,207:618−629. doi: 10.1016/j.jclepro.2018.10.040 [22] ZHANG K, LI Y, WANG Z, LI Q, WHIDDON R, HE Y, CEN K. Pyrolysis behavior of a typical Chinese sub-bituminous Zhundong coal from moderate to high temperatures[J]. Fuel,2016,185:701−708. doi: 10.1016/j.fuel.2016.08.038 [23] 路陈, 周志杰, 鑫刘, 帅袁, 王辅臣. 煤快速热解焦的微观结构对其气化活性的影响[J]. 燃料化学学报,2012,40(6):648−654. doi: 10.3969/j.issn.0253-2409.2012.06.002LU Chen, ZHOU Zhijie, XIN Liu, SHUAI Yuan, WANG Fuchen. Effect of microstructure of rapid pyrolysis char on its gasification reactivity[J]. J Fuel Chem Technol,2012,40(6):648−654. doi: 10.3969/j.issn.0253-2409.2012.06.002 [24] HE Y, CHANG C, LI P, HAN X, LI H, FANG S, CHEN J, MA X. Thermal decomposition and kinetics of coal and fermented cornstalk using thermogravimetric analysis[J]. Bioresour Technol,2018,259:294−303. doi: 10.1016/j.biortech.2018.03.043 [25] 李位位, 黄戒介, 王志青, 段会文, 李俊国, 房倚天. 煤焦CO2气化反应动力学及内扩散对气化过程的影响分析[J]. 燃料化学学报,2016,44(12):1416−1421. doi: 10.3969/j.issn.0253-2409.2016.12.002LI Weiwei, HUANG Jiejie, WANG Zhiqing, DUAN Huiwen, LI Junguo, FANG Yitian. Reaction kinetics of coal char gasification with CO2 and the effect of internal diffusion on the gasification[J]. J Fuel Chem Technol,2016,44(12):1416−1421. doi: 10.3969/j.issn.0253-2409.2016.12.002 [26] MIURA K, SILVESTON P L. Analysis of gas-solid reactions by use of a temperature-programmed reaction technique[J]. Energy Fuels,1989,3(2):243−249. doi: 10.1021/ef00014a020 [27] IWASZENKO S, HOWANIEC N, SMOLIŃSKI A. Determination of random pore model parameters for underground coal gasification simulation[J]. Energy,2019,166:972−978. doi: 10.1016/j.energy.2018.10.156 [28] GAO X, ZHANG Y, LI B, ZHAO Y, JIANG B. Determination of the intrinsic reactivities for carbon dioxide gasification of rice husk chars through using random pore model[J]. Bioresour Technol,2016,218:1073−1081. doi: 10.1016/j.biortech.2016.07.057 [29] KIM R-G, HWANG C-W, JEON C-H. Kinetics of coal char gasification with CO2: Impact of internal/external diffusion at high temperature and elevated pressure[J]. Appl Energy,2014,129:299−307. doi: 10.1016/j.apenergy.2014.05.011 [30] OLLERO P, SERRERA A, ARJONA R, ALCANTARILLA S. The CO2 gasification kinetics of olive residue[J]. Biomass Bioenergy,2003,24(2):151−161. doi: 10.1016/S0961-9534(02)00091-0 [31] JIANG L, ZHANG D, LI M, HE J-J, GAO Z-H, ZHOU Y, SUN J-H. Pyrolytic behavior of waste extruded polystyrene and rigid polyurethane by multi kinetics methods and Py-GC/MS[J]. Fuel,2018,222:11−20. doi: 10.1016/j.fuel.2018.02.143 [32] HE Q, GONG Y, DING L, GUO Q, YOSHIKAWA K, YU G. Reactivity prediction and mechanism analysis of raw and demineralized coal char gasification[J]. Energy,2021,229:120724. doi: 10.1016/j.energy.2021.120724 -




 下载:
下载: