Investigation of the surface acidity and redox on the CeO2-WO3 catalyst for selective catalytic reduction with NH3
-
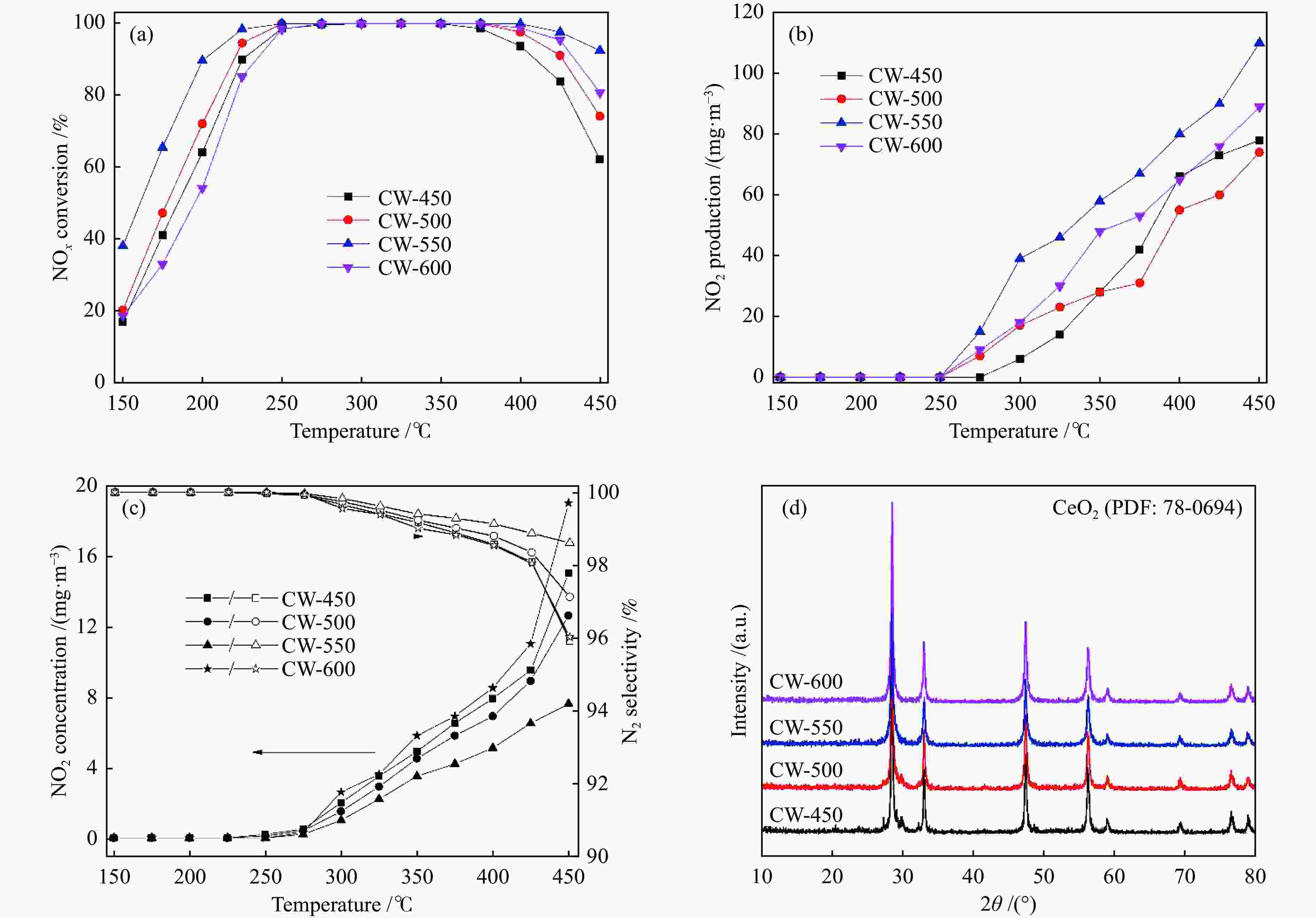
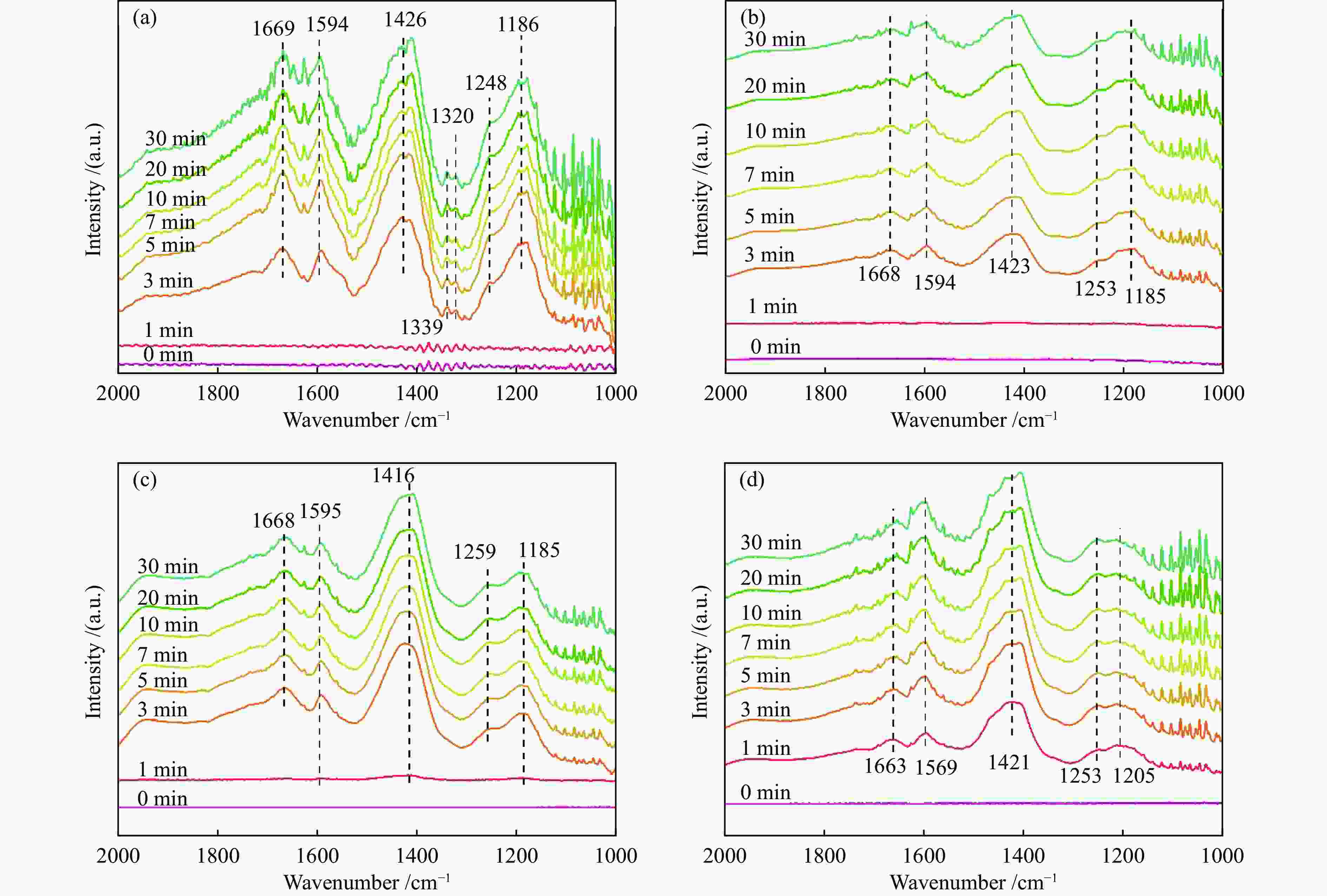
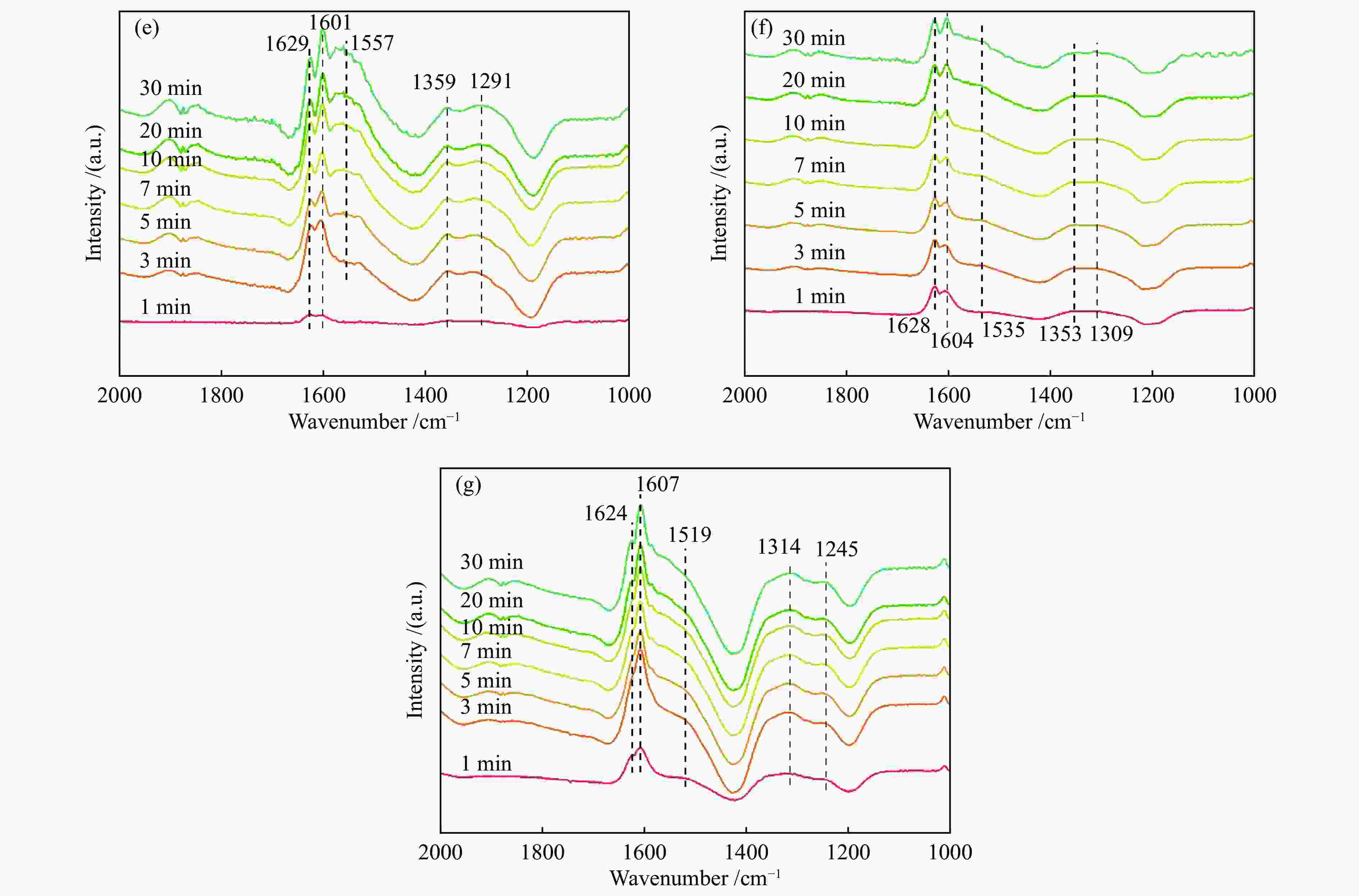
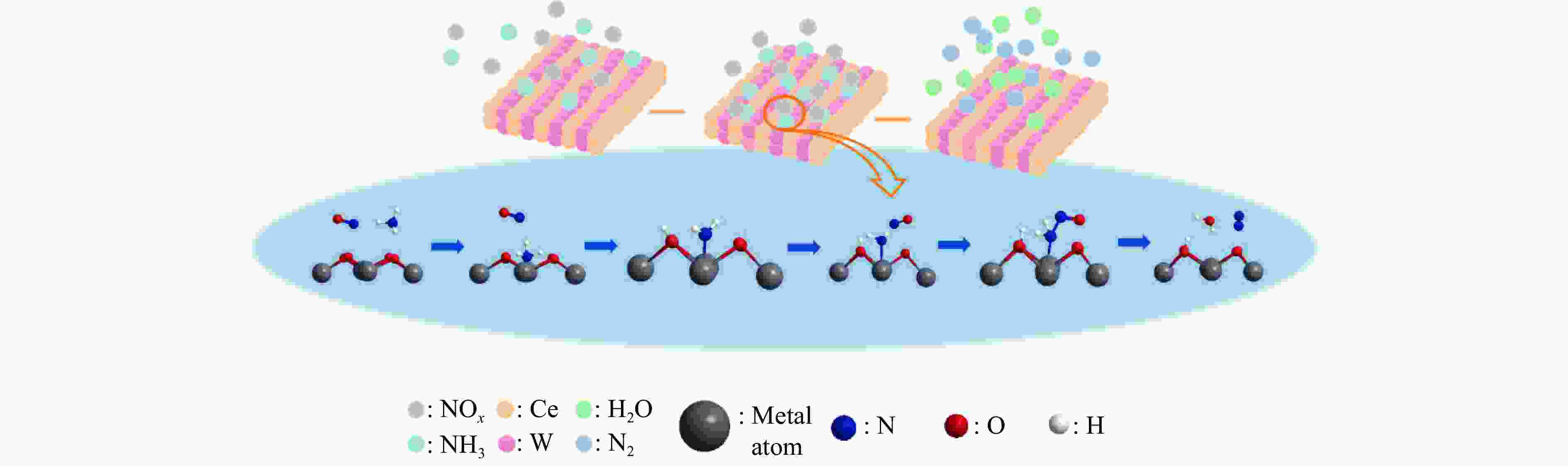
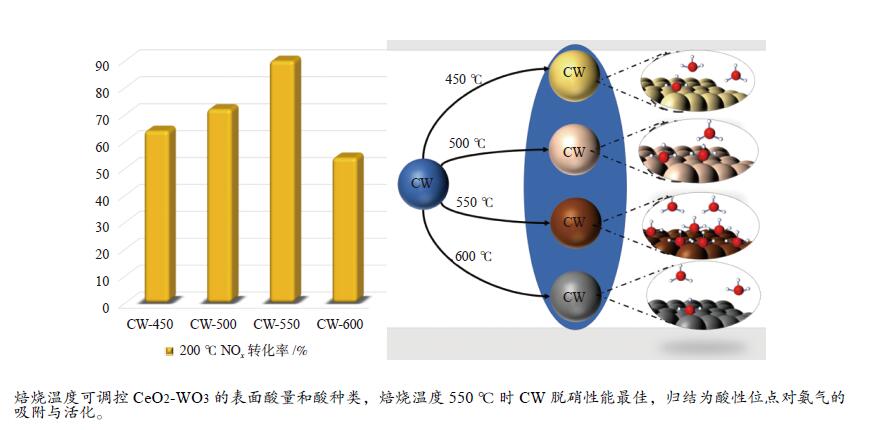
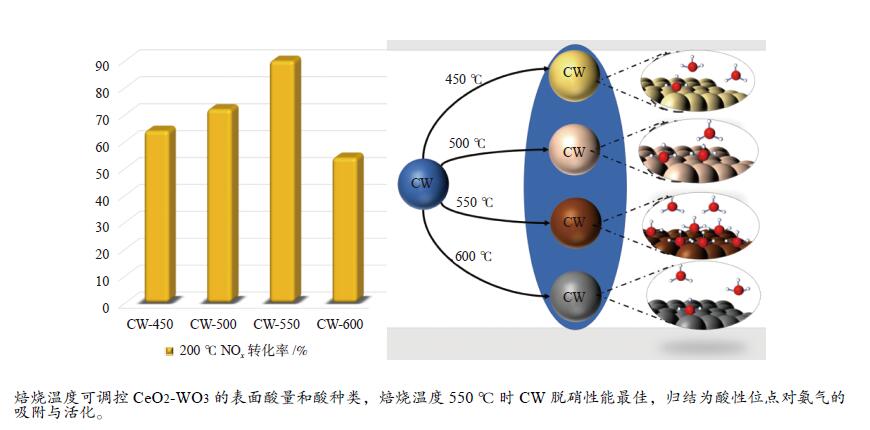
摘要: 本研究利用原位合成法成功制备CeO2-WO3催化剂并用于脱硝反应,焙烧温度为550 ℃的CW-550催化剂活性最佳,200 ℃时CW-550脱硝活性达到90%以上。CW-550催化剂具有优越的催化剂性能可归结为较大的比表面积、较多的Ce3 + 物种、丰富的表面酸性和优越的氧化还原性能。Ce3 + 增多,有利于氧空位的形成,可促进氧化还原性能。WO3的引入,在550 ℃的焙烧条件下可显著提升催化剂的Brönsted酸量,有利于氨气的吸附与活化,提升其催化性能。CW催化剂上吸附的NH3物种能与气态的NO反应,而吸附态的NH3与吸附态的NOx不能进行高效反应,因此,CW催化剂的SCR反应主要遵循Eley-Rideal反应机理。Abstract: CeO2-WO3 catalysts were successfully prepared by in-situ synthesis and used in the denitrification reaction. The best activity of CW-550 catalyst was achieved at a roasting temperature of 550 ℃, and the denitrification activity of CW-550 reached over 90% at 200 ℃. The superior catalytic performance of CW-550 catalyst can be attributed to the large specific surface area, more Ce3+ species, abundant surface acidity and superior redox performance. The increased Ce3+ facilitates the formation of oxygen vacancies and promotes redox performance. The introduction of WO3, into CeO2 can enhance the amounts of Brönsted acid, which contributes to the improvement of the adsorption and activation of ammonia, resulting in the excellent catalytic performance. The NH3-adsorbed species can react with gaseous NO. However, both of NH3-adsorbed and NO-adsorbed sepcies cannot participate in the SCR reaction effectively. Therefore, the SCR reaction of CW catalysts mainly follows the Eley-Rideal mechanism.
-
Key words:
- CeO2-WO3 /
- calcination temperature /
- denitration /
- surface acidity
-
表 1 表面原子比
Table 1 Surface atom concentration (%)
Sample Ce3 + /(Ce4 + + Ce3 + ) Oα/(Oα + Oβ) CW-450 20.64 25.76 CW-500 21.42 33.30 CW-550 23.94 16.81 CW-600 23.08 26.85 -
[1] SHAN W P, LIU F D, YU Y B, HE H, DENG C L, ZI X Y. High-efficiency reduction of NOx emission from diesel exhaust using a CeWOx catalyst[J]. Catal Commun,2015,59:226−228. doi: 10.1016/j.catcom.2014.10.032 [2] SHEN Z, LIU X Y, IMPENG S, ZHANG C B, YAN T T, WANG P L, ZHANG D S. Alkali and heavy metal copoisoning resistant catalytic reduction of NOx via liberating lewis acid sites[J]. Environ Sci Technol,2022,56(8):5141−5149. doi: 10.1021/acs.est.1c08096 [3] WANG P L, YAN L J, GU Y D, KUBOON S, LI H R, YAN T T, SHI L Y, ZHANG D S. Poisoning-resistant NOx reduction in the presence of alkaline and heavy metals over H-SAPO-34-supported Ce-promoted Cu-based catalysts[J]. Environ Sci Technol,2020,54(10):6396−6405. doi: 10.1021/acs.est.0c00100 [4] YAN L J, JI Y Y, WANG P L, FENG C, HAN L P, LI H R, YAN T T, SHI L Y, ZHANG D S. Alkali and phosphorus resistant zeolite-like catalysts for NOx reduction by NH3[J]. Environ Sci Technol,2020,54(14):9132−9141. doi: 10.1021/acs.est.0c03290 [5] ZHANG B L, ZHANG S G, LIU B. Effect of oxygen vacancies on ceria catalyst for selective catalytic reduction of NO with NH3[J]. Appl Surf Sci,2020,529:147068. doi: 10.1016/j.apsusc.2020.147068 [6] GENG Y, JIN K, MEI J, SU G Y, MA L, YANG S J. CeO2 grafted with different heteropoly acids for selective catalytic reduction of NOx with NH3[J]. J Hazard Mater,2020,382:121032. doi: 10.1016/j.jhazmat.2019.121032 [7] GHOLAMI F, TOMAS M, GHOLAMI Z, MOHAMMADTAGHI V. Technologies for the nitrogen oxides reduction from flue gas: A review[J]. Sci Total Environ,2020,714:136712. doi: 10.1016/j.scitotenv.2020.136712 [8] HU X L, CHEN J X, QU W Y, LIU R, XU D R, MA Z, TANG X F. Sulfur-resistant ceria-based low-temperature SCR catalysts with the non-bulk electronic states of ceria[J]. Environ Sci Technol,2021,55(8):5435−5441. doi: 10.1021/acs.est.0c08736 [9] JIANG S, LI T, ZHENG J K, ZHANG H, LI X, ZHU T L. Unveiling the remarkable arsenic resistance origin of alumina promoted cerium-tungsten catalysts for NH3-SCR[J]. Environ Sci Technol,2020,54(22):14740−14749. doi: 10.1021/acs.est.0c05152 [10] KANG L, HAN L P, HE J V, LI H R, YAN T T, CHEN G R, ZHANG J P, SHI L Y, ZHANG D S. Improved NOx reduction in the presence of SO2 by using Fe2O3-promoted halloysite-supported CeO2-WO3 catalysts[J]. Environ Sci Technol,2019,53(2):938−945. doi: 10.1021/acs.est.8b05637 [11] QI X R, HAN L P, DENG J, LAN T W, WANG F L, SHI L Y, ZHANG D S. SO2-tolerant catalytic reduction of NOx via tailoring electron transfer between surface iron sulfate and subsurface ceria[J]. Environ Sci Technol,2022,56(9):5840−5848. doi: 10.1021/acs.est.2c00944 [12] SONG Z X, LIU P, FU Y M, LIU H P, HUANG Z Z, KANG H Y, MAO Y L, LIU B, GUO Y F. Promotional effect of acidic oxide on catalytic activity and N2 selectivity over CeO2 for selective catalytic reduction of NOx by NH3[J]. Appl Organomet Chem,2019,33(6):e4919. doi: 10.1002/aoc.4919 [13] ZHANG Y P, WANG L F, LI J, ZHANG H Y, XU H T, XIAO R, YANG L J. Promotional roles of ZrO2 and WO3 in V2O5-WO3/TiO2-ZrO2 catalysts for NOx reduction by NH3: Catalytic performance, morphology, and reaction mechanism[J]. Chin J Catal,2016,37(11):1918−1930. doi: 10.1016/S1872-2067(16)62510-X [14] 李振壮, 熊志波, 何军飞, 李承绪, 屈小珂, 宁星, 吴水木, 陆威. 淀粉生物模板铈钨复合氧化物催化剂SCR脱硝性能[J]. 化学工程,2020,48(5):16−20. doi: 10.3969/j.issn.1005-9954.2020.05.004LI Zhen-zhuang, XIONG Zhi-bo, HE Jun-fei, LI Chen-xu, QU Xiao-ke, NING Xing, WU Shui-mu, LU Wei. Selective catalytic reduction of NOx of starch bio-template cerium-tungsten mixed oxide catalyst[J]. Chem Eng,2020,48(5):16−20. doi: 10.3969/j.issn.1005-9954.2020.05.004 [15] 李丽, 薛茹君, 陈春阳, 王庆超, 周敏, 陈淑芬. WO3(MoO3)-V2O5/CeO2-TiO2/HM的制备及其脱硝性能[J]. 合肥工业大学学报(自然科学版),2014,37(4):398−401.LI Li, XUE Ru-jun, CHEN Chun-yang, WANG Qing-chao, ZHOU Min, CHEN Shu-fen. Preparation of WO3(MoO3)-V2O5/CeO2-TiO2/HM and its denitration performance[J]. J Hefei Univ Technol,2014,37(4):398−401. [16] 马赫遥. 基于形貌调控的WO3/CeO2催化剂及其NH3-SCR脱硝机制[D]. 杭州: 浙江大学, 2020.MA He-yao. WO3/CeO2 catalyst and its NH3-SCR denitration mechanism based on morphology control[D]. Hangzhou: Zhejiang University, 2020. [17] CHEN L, LI J H, ABLIKIM W, WANG J, CHANG H Z, MA L, XU J Y, GE M F, ARANDIYAN H. CeO2-WO3 mixed oxides for the selective catalytic reduction of NOx by NH3 over a wide temperature range[J]. Catal Lett,2011,141(12):1859−1864. doi: 10.1007/s10562-011-0701-4 [18] WANG H, QU Z P, DONG S C, XIE H B, TANG C. Superior performance of Fe1−xWxOδ for the selective catalytic reduction of NOx with NH3: interaction between Fe and W[J]. Environ Sci Technol,2016,50(24):13511−13519. doi: 10.1021/acs.est.6b03589 [19] LI J Y, SONG Z X, NING P, ZHANG Q L, LIU X, LI H, HUANG Z Z. Influence of calcination temperature on selective catalytic reduction of NOx with NH3 over CeO2-ZrO2-WO3 catalyst[J]. J Rare Earth,2015,33(7):726−735. doi: 10.1016/S1002-0721(14)60477-4 [20] 王栋, 张信莉, 彭建升, 路春美, 韩奎华, 徐丽婷. 煅烧温度对γ-Fe2O3催化剂结构及其脱硝活性的影响[J]. 环境科学研究,2015,28(5):808−815.WANG Dong, ZHANG Xin-li, PENG Jian-sheng, LU Chun-mei, HAN Kui-hua, XU Li-ting. Effect of calcination temperature on selective catalytic reduction of NOx over γ-Fe2O3 catalyst prepared with microwave assistance[J]. Res Environ Sci,2015,28(5):808−815. [21] 陈邱谆, 丁凯, 路春美, 巩志强, 吕泽康. 煅烧温度对铈改性赤泥催化剂脱硝性能的影响[J]. 广州化工,2020,48(21):46−49. doi: 10.3969/j.issn.1001-9677.2020.21.019CHEN Qiu-zhun, DING Kai, LU Chun-mei, GONG Zhi-qiang, LU Ze-kang. Effect of calcination temperature on selective catalytic reduction of cerium-modified red mud catalyst[J]. Guangzhou Chem Ind,2020,48(21):46−49. doi: 10.3969/j.issn.1001-9677.2020.21.019 [22] 张信莉, 王栋, 彭建升, 路春美, 徐丽婷. 煅烧温度对Mn改性γ-Fe2O3催化剂结构及低温SCR脱硝活性的影响[J]. 燃料化学学报,2015,43(2):243−250. doi: 10.3969/j.issn.0253-2409.2015.02.016ZHANG Xin-li, WANG Dong, PENG Jian-sheng, LU Chun-mei, XU Li-ting. Influence of calcination temperature on structural of Mn-doped γ-Fe2O3 catalysts and low temperature SCR activity[J]. J Fuel Chem Technol,2015,43(2):243−250. doi: 10.3969/j.issn.0253-2409.2015.02.016 [23] MA S Y, GAO W Q, YANG Z D, LIN R Y, WANG X W, ZHU X B, JIANG Y. Superior Ce-Nb-Ti oxide catalysts for selective catalytic reduction of NO with NH3[J]. J Energy Inst,2021,94:73−84. doi: 10.1016/j.joei.2020.11.001 [24] 李露露. 铈基NH3-SCR催化剂的制备及其脱硝性能研究[D]. 南京: 南京大学, 2017.LI Lu-lu. The research of preparation and performance of ceria-based catalyst in the NH3-selective catalytic reduction[D]. Nanjing: Nanjing University, 2017. [25] ZHAN S H, ZHANG H, ZHANG Y, SHI Q, LI Y, LI X J. Efficient NH3-SCR removal of NOx with highly ordered mesoporous WO3(chi)-CeO2 at low temperatures[J]. Appl Catal B: Environ,2017,203:199−209. doi: 10.1016/j.apcatb.2016.10.010 [26] 田俊奇. Mn基催化剂的制备及其在低温脱硝中的应用[D]. 石河子: 石河子大学, 2020.TIAN Jun-qi. Preparation of Mn-based catalyst and its application in Low temperature denitration[D]. Shihezi: Shihezi University, 2020. [27] 罗贵莹. Ce-W脱硝催化剂的研究[D]. 北京: 北京化工大学, 2020.LUO Gui-ying. Study on Ce-W catalyst for the NH3-SCR of NOx[D]. Beijing: Beijing University of Chemical Technology, 2020. [28] XIAO X X, WANG J J, JIA X F, MA C, QIAO W M, LING L C. Low-temperature selective catalytic reduction of NOx with NH3 over Mn-Ce composites synthesized by polymer-assisted deposition[J]. ACS Omega,2021,6(19):12801−12812. doi: 10.1021/acsomega.1c01123 [29] CHOUDHURY B. CHOUDHURY A. Ce3 + and oxygen vacancy mediated tuning of structural and optical properties of CeO2 nanoparticles[J]. Mater Chem Phys,2012,131(3):666−671. doi: 10.1016/j.matchemphys.2011.10.032 [30] ZHENG L R, LIU C, TAN Y S, WANG L H, XIAN H. Insight into the improvement effect of the Ce doping into the SnO2 catalyst for the catalytic combustion of methane[J]. Appl Catal B: Environ,2015,176:542−552. [31] LI X, LI X S, LI J H, HAO J M. High calcium resistance of CeO2-WO3 SCR catalysts: Structure investigation and deactivation analysis[J]. Chem Eng J,2017,317:70−79. doi: 10.1016/j.cej.2017.02.027 [32] MA Z R, WENG D, WU X D, SI Z C. Effects of WOx modification on the activity, adsorption and redox properties of CeO2 catalyst for NOx reduction with ammonia[J]. J Environ Sci,2012,24(7):1305−1316. doi: 10.1016/S1001-0742(11)60925-X [33] LI X L, WANG Z, SUN J, OH R, FENG J J, SHI D D, ZHAO W, LIU S S. Influence of CeO2 morphology on WO3/CeO2 catalyzed NO selective catalytic reduction by NH3[J]. J Energy Inst,2020,93(4):1511−1518. doi: 10.1016/j.joei.2020.01.013 [34] PENG Y, LI K Z, LI J H. Identification of the active sites on CeO2-WO3 catalysts for SCR of NOx with NH3: An in situ IR and Raman spectroscopy study[J]. Appl Catal B: Environ, 2013, 140−141, 483−492. [35] 王丹. 铈基复合氧化物催化剂的优化及脱硝性能研究[D]. 大连: 大连理工大学, 2018.WANG Dan. Study on optimization of cerium based mixed oxide catalysts and their denitrification performance[D]. Dalian: Dalian University of Technology, 2018. [36] 王俊凯. 无机非金属酸(SO42−/PO43−)改性铈基催化剂脱硝性能研究[D]. 沈阳: 沈阳化工大学, 2019.WANG Jun-kai. Inorganic nonmetallic acid (SO42−/PO43−) modified of CeO2 catalyst for denitration performance[D]. Shenyang: Shenyang University of Chemical Technology, 2019. [37] 王雪冲. 碱(土)金属对Ce基催化剂SCR烟气脱硝性能的影响研究[D]. 上海: 中国石油大学(东华), 2017.WANG Xue-chong. The effect of alkali and alkaline earth metal on Ce based catalysts selective catalytic reduction of NO[D]. Shanghai: China University of Petroleum (Donghua), 2017. [38] ZHANG L. SUN J F, XIONG Y, ZENG X Q, TANG C J, DONG L. Catalytic performance of highly dispersed WO3 loaded on CeO2 in the selective catalytic reduction of NO by NH3[J]. Chin J Catal,2017,38(10):1749−1758. doi: 10.1016/S1872-2067(17)62887-0 [39] WANG D, PENG Y, YANG Q L, HUA F Y, LI J H, CRITTENDEN J. NH3-SCR performance of WO3 blanketed CeO2 with different morphology: balance of surface reducibility and acidity[J]. Catal Today,2019,332:42−48. doi: 10.1016/j.cattod.2018.07.048 [40] 訾朝辉. Mn改性泡沫镍负载铁基催化剂低温SCR脱硝性能研究[D]. 马鞍山: 安徽工业大学, 2019.ZI Zhao-hui. Low-temperature SCR deNOx performance of Mn modified foamed nickel supported iron-based catasts[D]. Maanshan: Anhui University of Technology, 2019. [41] LI X, LI X S, ZHU T L, PENG Y, LI J H, HAO J M. Extraordinary deactivation offset effect of arsenic and calcium on CeO2-WO3 SCR catalysts[J]. Environ Sci Technol,2018,52(15):8578−8587. doi: 10.1021/acs.est.8b00746 [42] PENG Y. QU R Y, ZHANG X Y, LI J H. The relationship between structure and activity of MoO3-CeO2 catalysts for NO removal: influences of acidity and reducibility[J]. Chem Commun,2013,49(55):6215−7. doi: 10.1039/c3cc42693a [43] HE G Z. GAO M, PENG Y, YU Y B, SHAN W P, HE H. Superior oxidative dehydrogenation performance toward NH3 determines the excellent low-temperature NH3-SCR activity of Mn-based catalysts[J]. Environ Sci Technol,2021,55(10):6995−7003. doi: 10.1021/acs.est.0c08214 -




 下载:
下载: