Preparation of PdAg/CDs-ZSM-5 catalyst and performance study on furfural aqueous phase hydrogenation-rearrangement to cyclopentanone
-
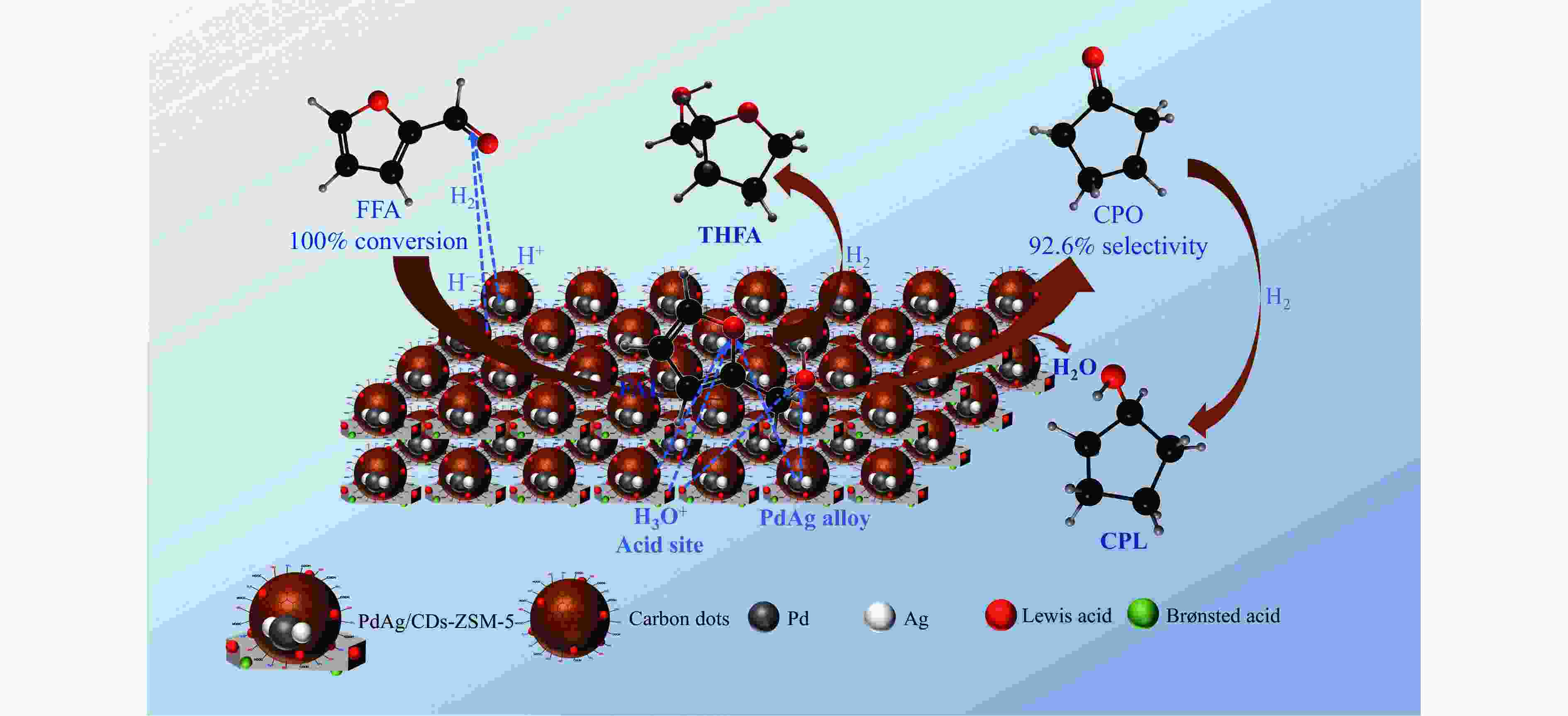
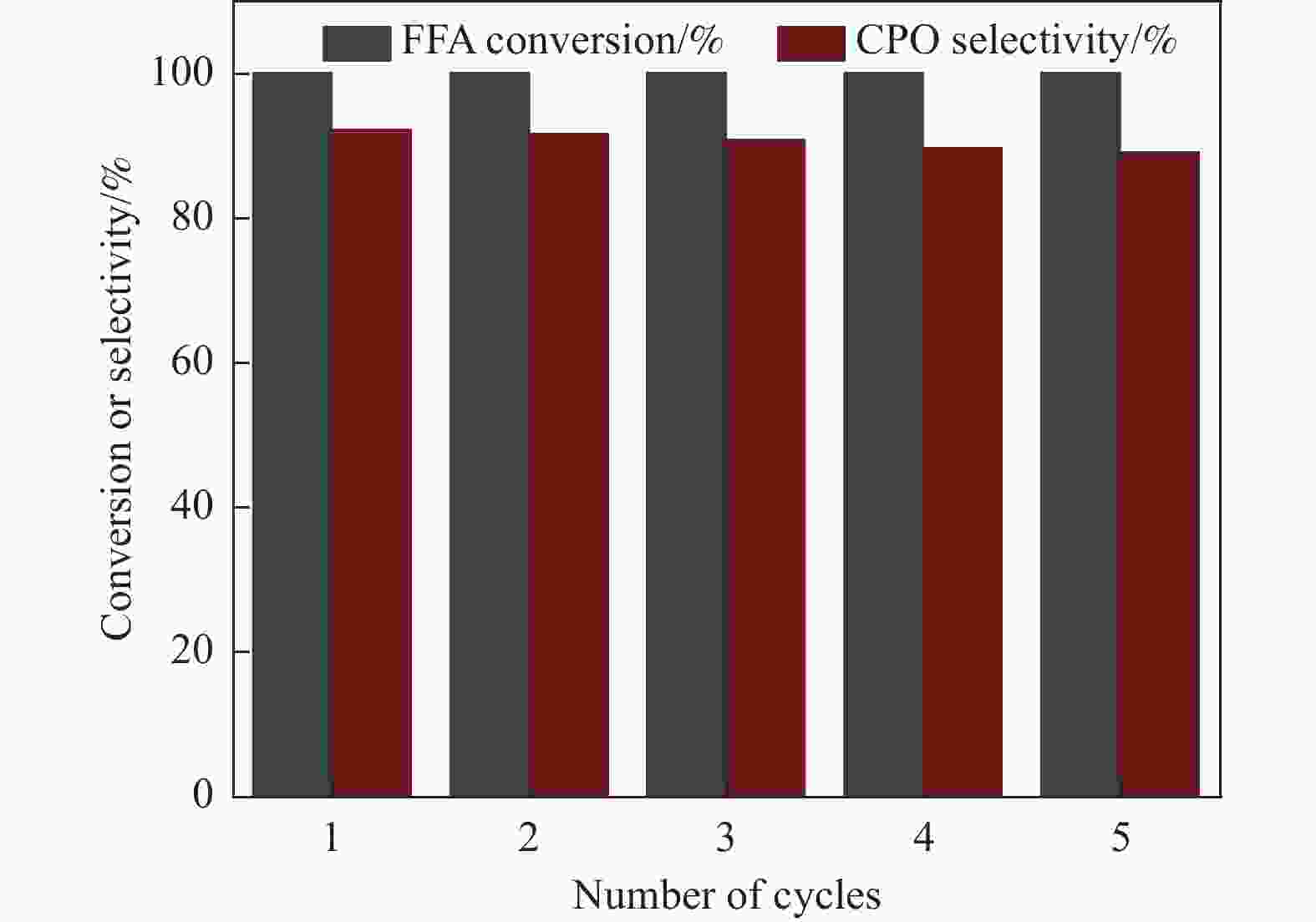
摘要: 以沸石分子筛(ZSM-5)为载体,碳点(carbon dots, CDs)为还原剂和稳定剂,通过光照还原法制备了双金属PdAg/CDs-ZSM-5催化剂,用于糠醛(furfural, FFA)水相加氢-重排制备环戊酮(cyclopentanone, CPO)反应。采用X射线衍射(XRD)、X射线光电子能谱(XPS)、扫描电子显微镜(SEM)、透射电子显微镜(TEM)、氨气程序化学吸附(NH3-TPD)和吡啶红外(Py-FTIR)等手段对催化剂进行了表征。结果表明,CDs具有良好的还原性和丰富的Lewis酸性位点,能够将Pd2+、Ag+还原为金属单质并形成纳米合金结构,复合催化剂中适宜的酸性位点与PdAg合金之间的协同作用使得PdAg/CDs-ZSM-5催化剂在最优反应条件下,对FFA转化率达到100%,目标产物CPO选择性为92.6%。催化剂重复使用五次后仍能保持较高的活性与稳定性。
-
关键词:
- 碳点 /
- PdAg/CDs-ZSM-5 /
- 糠醛 /
- Lewis酸性位点 /
- 环戊酮
Abstract: With the huge demand for fossil resources and increasing energy consumption, the utilization of biomass as a renewable alternative to the production of chemicals and fuels has attracted much attention. Furfural (FFA), as an important biomass-based derived carbonyl compound, can be industrially produced on a large scale from lignocellulosic biomass feedstocks and converted into various high-value chemicals, liquid fuels, and functional materials through a variety of pathways, which is crucial for alleviating the global fossil resource crisis and achieving carbon peaking and carbon neutrality goals. Carbon dots (CDs) are a new type of zero-dimensional carbon-based nanomaterials with particle size usually less than 10 nm, whose core is usually composed of sp2 hybridized carbon, and whose surface contains abundant functional groups such as hydroxyl, amino, and carboxyl groups, which have excellent UV-visible absorption, strong proton adsorption, and good stability and hydrophilicity. The high electron-transferring property of the surface functional groups of CDs makes them excellent electron carriers and donors, especially under UV light irradiation. Especially under the irradiation of ultraviolet light, CDs can be used as a reducing agent and stabilizer to reduce metal ions to metal monomers. Zeolite molecular sieves can effectively promote the diffusion of reactants and products in the pores and improve the catalytic activity due to their highly ordered pores, large specific surface area, suitable pore size and good hydrothermal stability. Therefore, molecular sieves can be a good choice of carrier in multiphase catalysis, and their unique domain-limited environment can provide spatial confinement for metal particles to improve the resistance of metals to sintering and prevent the leaching of active metal substances during the catalytic process. Based on this, in this paper, bimetallic PdAg/CDs-ZSM-5 catalysts were prepared by reduction with zeolite molecular sieve ZSM-5 as the carrier and CDs as the reducing and stabilizing agents via UV irradiation and applied to the aqueous-phase hydrogenation-rearrangement of FFA for the preparation of cyclopentanone (CPO) reaction. The CDs and composite catalysts were characterized by X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and pyridine infrared (Py-FTIR). The results showed that the CDs had good reducibility and abundant Lewis acidic sites, and were able to reduce Pd2+ and Ag+ to metal monomers and form PdAg nano-alloy structures. The effects of reaction temperature, reaction time and hydrogen pressure on the reaction performance of the aqueous-phase selective hydrogenation-rearrangement of FFA to produce CPO were investigated using PdAg/CDs-ZSM-5 as catalyst. It was shown that the synergistic effect between the suitable acidic sites on the composite catalyst and the PdAg alloy greatly promoted the rearrangement of the reaction intermediate FAL, thus selectively controlling the hydrogenation of FFA to produce FAL first and then further rearrangement to obtain CPO. 100% conversion of FFA was achieved at the reaction temperature of 160 ℃, 2 MPa H2, and the target product CPO under the reaction conditions of 4 h. The selectivity of the target product CPO was 92.6%. selectivity was 92.6%. After reusing the catalyst for 5 times, the conversion of FFA was basically unchanged, and the selectivity of CPO only decreased by 3.6 %.-
Key words:
- carbon dots /
- PdAg/CDs-ZSM-5 /
- furfural /
- Lewis acidic site /
- cyclopentanone
-
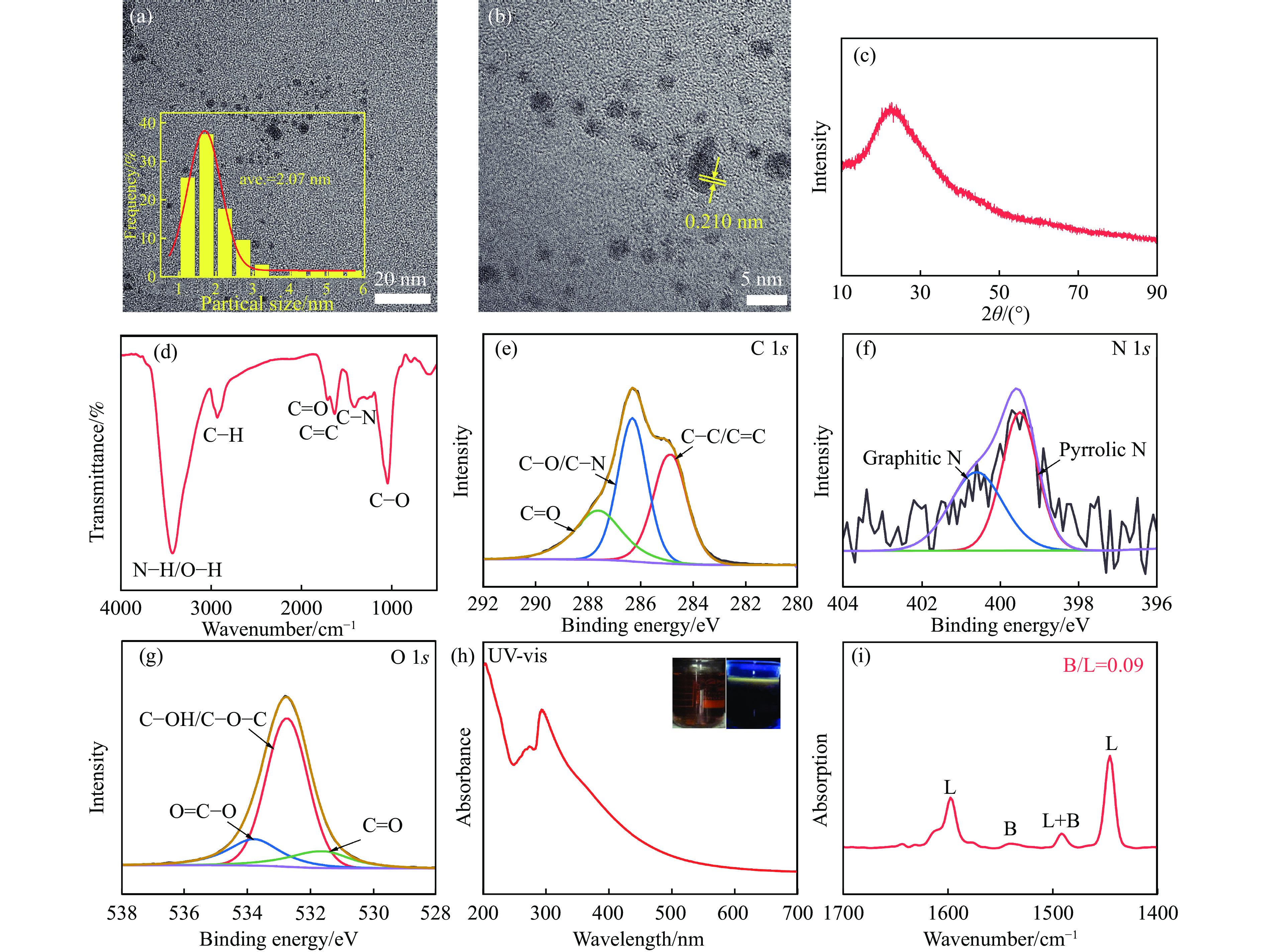
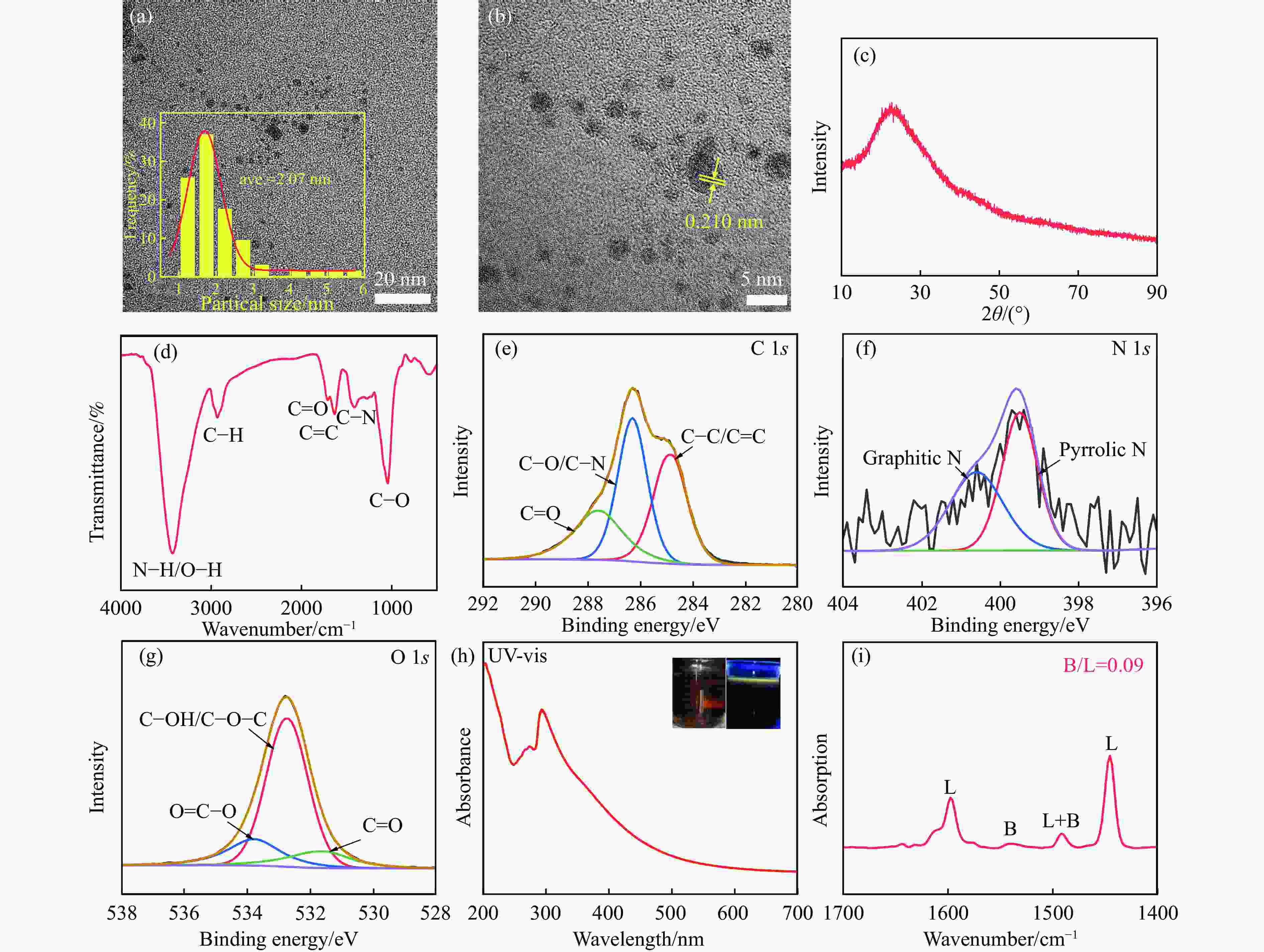
图 1 CDs的(a) TEM图像;(b) HRTEM 图像;(c) XRD谱图;(d) FT-IR谱图;(e) C 1s、(f) N 1s和(g) O 1s的高分辨率XPS谱图;(h) UV-vis光谱谱图以及(i) Py-FTIR谱图
Figure 1 CDs of (a) TEM image; (b) HRTEM image; (c) XRD spectra; (d) infrared spectra; high-resolution XPS of (e) C 1s, (f) N 1s, and (g) O 1s; (h) UV-vis spectra and (i) Py-FTIR spectra
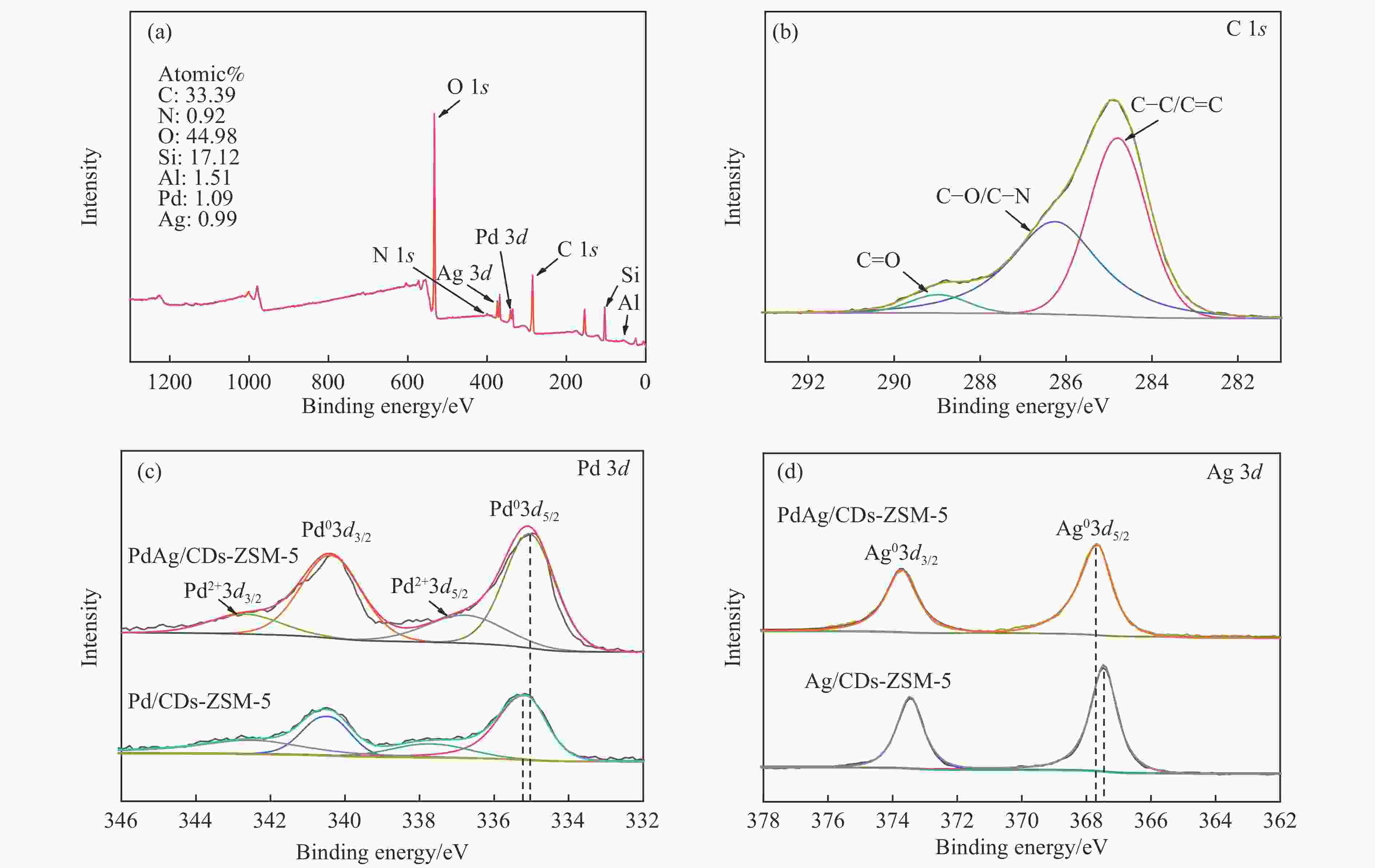
图 5 (a)PdAg/CDs-ZSM-5的XPS全谱图;(b)PdAg/CDs-ZSM-5的C 1s精细谱图;(c)PdAg/CDs-ZSM-5和Pd/CDs-ZSM-5的Pd 3d精细谱图;(d)PdAg/CDs-ZSM-5和Ag/CDs-ZSM-5 的Ag 3d精细谱图
Figure 5 (a) XPS Full spectrum of PdAg/CDs-ZSM-5 and high-resolution spectra for (b) C 1s of PdAg/CDs-ZSM-5; (c) Pd 3d of PdAg/CDs-ZSM-5 and Pd/CDs-ZSM-5; (d) Ag 3d of PdAg/CDs-ZSM-5 and Ag/CDs-ZSM-5
表 1 ZSM-5和PdAg/CDs-ZSM-5的微观结构
Table 1 Comparison of microscopic structure parameters of the ZSM-5 and PdAg/CDs-ZSM-5
Sample BET surface area/(m2·g−1) Pore volume/(cm3·g−1) Pore diameter/nm ZSM-5 374 0.19 2.02 PdAg/CDs-ZSM-5 298 0.15 2.07 表 2 不同催化剂上FFA加氢及重排的转化率及产物选择性
Table 2 Conversion and products selectivity of FFA hydrogenation and rearrangement over different catalysts a
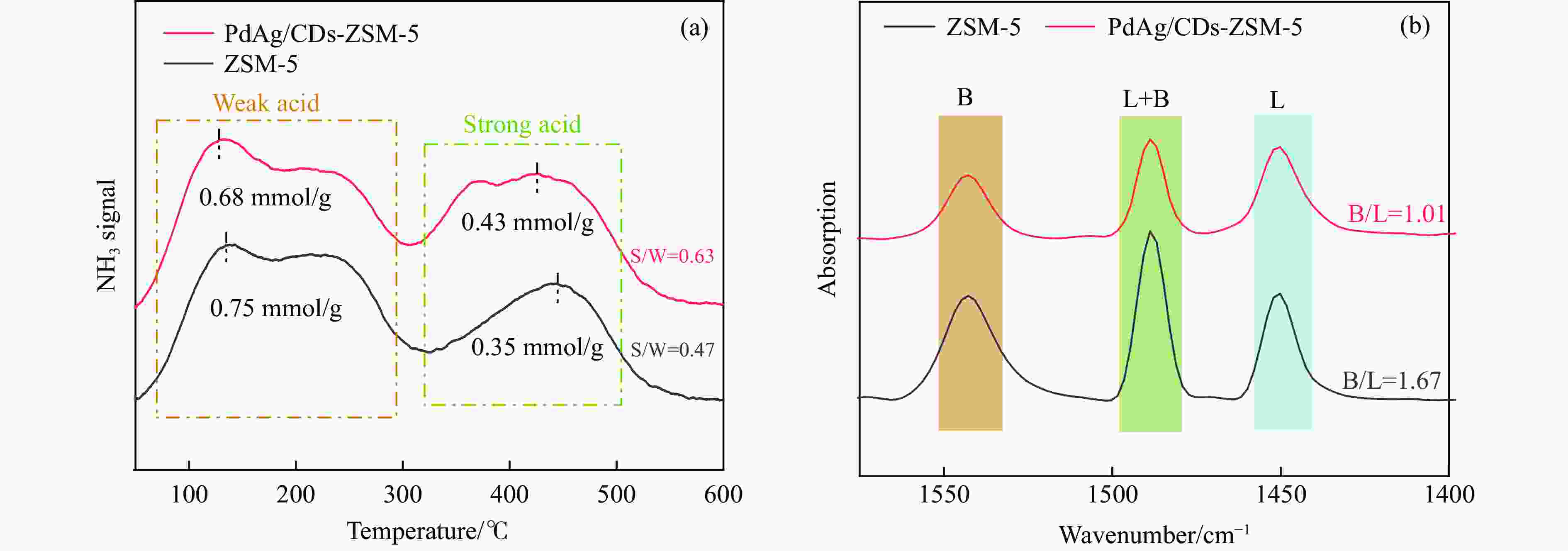
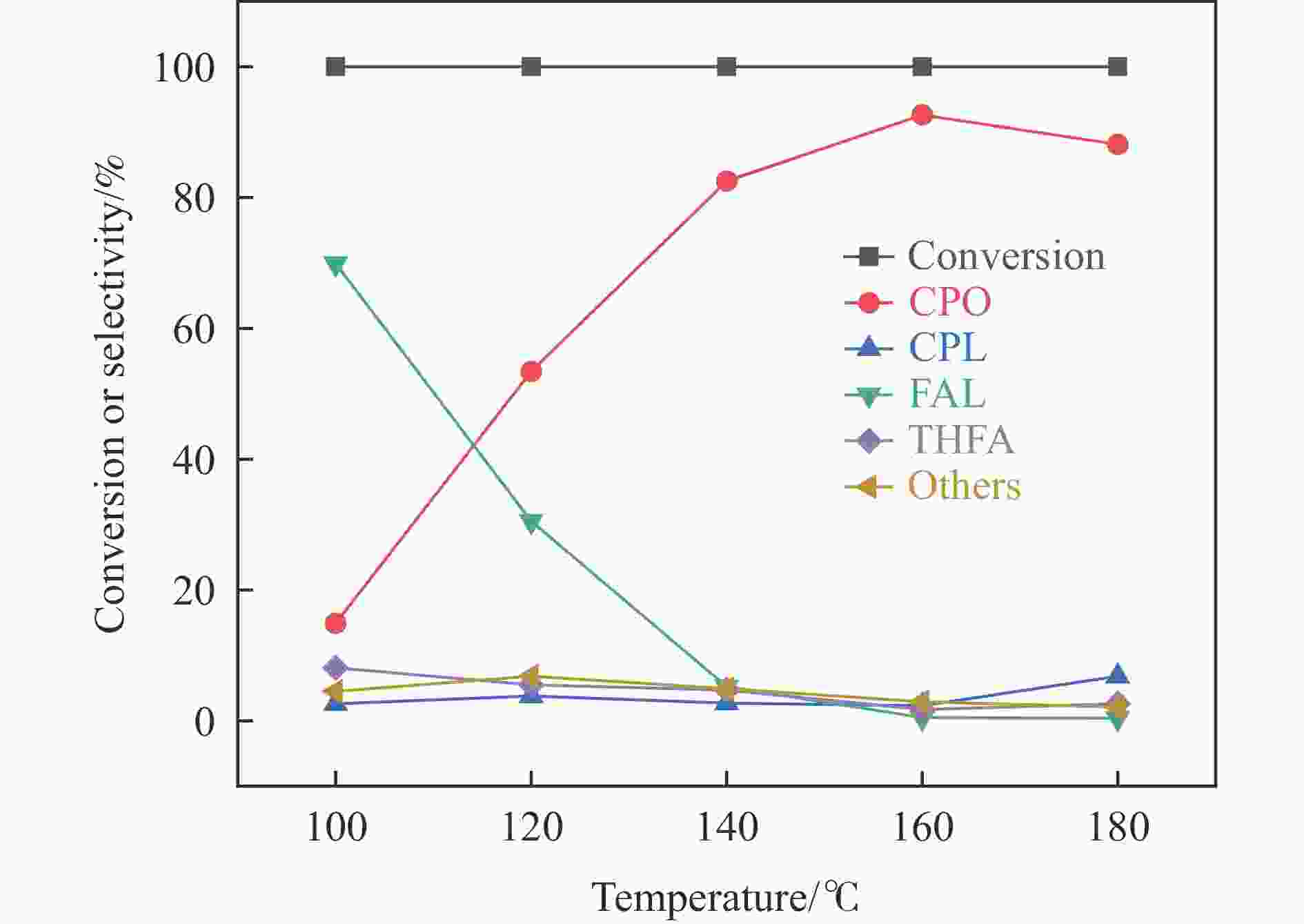
Entry Catalyst Conversion/% Selectivity/% C balance/% FAL THFA CPO CPL 1 ZSM-5 11.3 53.8 2.1 39.7 − 96 2 Ag/CDs-ZSM-5 43.6 83.4 4.8 10.5 0.8 99 3 Pd/CDs-ZSM-5 97.2 0.3 21.5 67.1 6.5 96 4 PdAg/CDs-ZSM-5 100 0.5 1.7 92.6 2.3 97 5 Pd/CDs-ZSM-5+ Ag/CDs-ZSM-5 82.9 17.8 16.5 56.8 5.2 96 a: Conditions: 2 mmol FFA, 100 mg catalysts, 160 ℃, 2 MPa H2, 4 h, 30 mL H2O. 表 3 ICP-OES测定的不同催化剂中金属的含量
Table 3 Determination of Metal content in different Catalysts by ICP-OES
Catalysts Pd loading w/% Ag loading w/% Pd/CDs-ZSM-5 8.93 − Ag/CDs-ZSM-5 − 8.72 PdAg/CDs-ZSM-5 4.51 4.47 PdAg/CDs-ZSM-5(5 cycles) 4.37 4.29 -
[1] BOHRE A, ALAM M I, AVASTHI K, et al. low temperature transformation of lignocellulose derived bioinspired molecules to aviation fuel precursor over magnesium-lanthanum mixed oxide catalyst[J]. Appl Catal B: Environ,2020,276:12. [2] YUAN E X, WANG C L, WU C, et al. constructing hierarchical structures of pd catalysts to realize reaction pathway regulation of furfural hydroconversion[J]. J Catal,2023,421:30−44. doi: 10.1016/j.jcat.2023.03.009 [3] LIN W, ZHANG Y X, MA Z X, et al. synergy between ni3sn2 alloy and lewis acidic reox enables selectivity control of furfural hydrogenation to cyclopentanone[J]. Appl Catal B: Environ,2024,340:11. [4] YANG Y L, DU Z T, HUANG Y Z, et al. conversion of furfural into cyclopentanone over ni-cu bimetallic catalysts[J]. Green Chem,2013,15(7):1932−1940. [5] MIRONENKO R M, BELSKAYA O B, TALSI V P, et al. mechanism of pd/c-catalyzed hydrogenation of furfural under hydrothermal conditions[J]. J Catal,2020,389:721−734. doi: 10.1016/j.jcat.2020.07.013 [6] HU Z, XIE A D, CHEN C, et al. facile synthesis of n, p co-doped carbon encapsulated ni catalyst for green production of cyclopentanone from biomass derivative furfural[J]. Fuel,2022,319:9. [7] YOON Y, ROUSSEAU R, WEBER R S, et al. first-principles study of phenol hydrogenation on pt and ni catalysts in aqueous phase[J]. J Am Chem Soc,2014,136(29):10287−10298. [8] DENG Q, GAO R, LI X, et al. hydrogenative ring-rearrangement of biobased furanic aldehydes to cyclopentanone compounds over pd/pyrochlore by introducing oxygen vacancies[J]. ACS Catal,2020,10(13):7355−7366. doi: 10.1021/acscatal.0c01666 [9] PHAM H N, ANDERSON A E, JOHNSON R L, et al. improved hydrothermal stability of mesoporous oxides for reactions in the aqueous phase[J]. Angew Chem-Int Edit,2012,51(52):13163−13167. [10] HUO J J, TESSONNIER J P, SHANKS B H. improving hydrothermal stability of supported metal catalysts for biomass conversions: a review[J]. ACS Catal,2021,11(9):5248−5270. doi: 10.1021/acscatal.1c00197 [11] GAO J, ZHU M M, HUANG H, et al. advances, challenges and promises of carbon dots[J]. Inorg Chem Front,2017,4(12):1963−1986. doi: 10.1039/C7QI00614D [12] YAO B W, HUANG H, LIU Y, et al. carbon dots: A small conundrum[J]. Trends Chem,2019,1(2):235−246. [13] KANG Z H, LEE S T. carbon dots: advances in nanocarbon applications[J]. Nanoscale,2019,11(41):19214−19224. [14] HAN Y D, WU J, LI Y, et al. carbon dots enhance the interface electron transfer and photoelectrochemical kinetics in tio2 photoanode[J]. Appl Catal B: Environ,2022,304:10. [15] LIU Y M, ROY S, SARKAR S, et al. a review of carbon dots and their composite materials for electrochemical energy technologies[J]. Carbon Energy,2021,3(5):795−826. [16] LU S Y, XIAO G J, SUI L Z, et al. piezochromic carbon dots with two-photon fluorescence[J]. Angew Chem-Int Edit,2017,56(22):6187−6191. doi: 10.1002/anie.201700757 [17] BAYAN R, KARAK N. photo-assisted synthesis of a pd-ag@cqd nanohybrid and its catalytic efficiency in promoting the suzuki-miyaura cross-coupling reaction under ligand-free and ambient conditions[J]. ACS Omega,2017,2(12):8868−8876. [18] DEY D, BHATTACHARYA T, MAJUMDAR B, et al. carbon dot reduced palladium nanoparticles as active catalysts for carbon-carbon bond formation[J]. Dalton Trans,2013,42(38):13821−13825. doi: 10.1039/c3dt51234g [19] MURUGESAN P, LIBIYA N, MOSES J A, et al. fluorescence resonance energy transfer-based sensor with silver-conjugated orange peel waste-derived carbon dots for melamine detection[J]. Carbon Letters,2023,33(7):2335−2348. [20] ZHANG J, CHEN Y, TAN J, et al. the synthesis of rhodium/carbon dots nanoparticles and its hydrogenation application[J]. Appl Surf Sci,2017,396:1138−1145. doi: 10.1016/j.apsusc.2016.11.101 [21] LI W D, WEI Z H, WANG B Y, et al. carbon quantum dots enhanced the activity for the hydrogen evolution reaction in ruthenium-based electrocatalysts[J]. Mat Chem Front,2020,4(1):277−284. doi: 10.1039/C9QM00618D [22] DUAN Y, HUANG Y J, CHEN S Y, et al. cu-doped carbon dots as catalysts for the chemiluminescence detection of glucose[J]. ACS Omega,2019,4(6):9911−9917. doi: 10.1021/acsomega.9b00738 [23] GUO S J, ZHAO S Q, GAO J, et al. cu-cdots nanocorals as electrocatalyst for highly efficient co2 reduction to formate[J]. Nanoscale,2017,9(1):298−304. [24] LI W D, LIU Y, WU M, et al. carbon-quantum-dots-loaded ruthenium nanoparticles as an efficient electrocatalyst for hydrogen production in alkaline media[J]. Adv Mater,2018,30(31):8. [25] LIU Y P, LEI J H, WANG G, et al. toward strong near-infrared absorption/emission from carbon dots in aqueous media through solvothermal fusion of large conjugated perylene derivatives with post-surface engineering[J]. Adv Sci,2022,9(23):11. [26] LU S Y, SUI L Z, LIU J J, et al. near-infrared photoluminescent polymer-carbon nanodots with two-photon fluorescence[J]. Adv Mater,2017,29(15):6. [27] CHANG Q, YANG S S, XUE C R, et al. nitrogen-doped carbon dots encapsulated in the mesoporous channels of sba-15 with solid-state fluorescence and excellent stability[J]. Nanoscale,2019,11(15):7247−7255. [28] JOKAR F, ALAVI S M, REZAEI M. investigating the hydroisomerization of n-pentane using pt supported on zsm-5, desilicated zsm-5, and modified zsm-5/mcm-41[J]. Fuel,2022,324:11. [29] WU Z L, WANG J, WANG S, et al. controllable chemoselective hydrogenation of furfural by pdag/c bimetallic catalysts under ambient operating conditions: an interesting ag switch[J]. Green Chem,2020,22(4):1432−1442. [30] CORREGIDOR P F, CUESTA P M, ACOSTA D E, et al. composite zsm-5/mcm-41 material obtained from a green resource and its enhanced catalytic performance in the reaction of vinyl acetate and isoamyl alcohol[J]. Appl Catal A-Gen,2019,587:11. [31] TAMIZHDURAI P, KRISHNAN P S, RAMESH A, et al. isomerization of hydrocarbons over pt supported on micro-mesoporous zsm-5[J]. Polyhedron,2018,154:314−324. [32] FU L C, XIONG Q A, WANG Q H, et al. catalytic pyrolysis of waste polyethylene using combined cao and ga/zsm-5 catalysts for high value-added aromatics production[J]. ACS Sustain Chem Eng,2022,10(29):9612−9623. doi: 10.1021/acssuschemeng.2c02881 [33] BAO K L, LIAO F, ZHOU Y J, et al. carbon dots regulate the multiple-proton-electron catalytic process and switch carbon dioxide reduction to hydrogen evolution reaction of pdcu alloy electrocatalyst[J]. Appl Surf Sci,2023,619:9. [34] YANG G X, KUWAHARA Y, MORI K, et al. pdag alloy nanoparticles encapsulated in n-doped microporous hollow carbon spheres for hydrogenation of co2 to formate[J]. Appl Catal B: Environ,2021,283:13. [35] YANG G X, YIN H B, LIU W H, et al. synergistic ag/tio2-n photocatalytic system and its enhanced antibacterial activity towards acinetobacter baumannii[J]. Appl Catal B: Environ,2018,224:175−182. [36] WANG X J, ZHANG R Y, MA X Y, et al. carbon dots@noble metal nanoparticle composites: research progress report[J]. Analyst,2024,149(3):665−688. doi: 10.1039/D3AN01580G [37] LIU H, LIU X Y, YANG W W, et al. photocatalytic dehydrogenation of formic acid promoted by a superior pdag@g-c3/n4 mott-schottky heterojunction[J]. J Mater Chem A,2019,7(5):2022−2026. doi: 10.1039/C8TA11172C [38] ZHANG Z J, LUO Y X, LIU S W, et al. a pdag-ceo2 nanocomposite anchored on mesoporous carbon: A highly efficient catalyst for hydrogen production from formic acid at room temperature[J]. J Mater Chem A,2019,7(37):21438−21446. doi: 10.1039/C9TA06987A [39] ZHANG Y F, FAN G L, YANG L, et al. efficient conversion of furfural into cyclopentanone over high performing and stable cu/zro2 catalysts[J]. Appl Catal A-Gen,2018,561:117−126. [40] GAO X, DING Y Y, PENG L L, et al. on the effect of zeolite acid property and reaction pathway in pd-catalyzed hydrogenation of furfural to cyclopentanone[J]. Fuel,2022,314:9. -




 下载:
下载: